-
环孢素(cyclosporine A, CsA)是肾移植术后三联免疫抑制方案中的基础用药之一。环孢素个体差异大、治疗窗窄,静脉给药虽能更快达到目标浓度,但也更容易使血药浓度偏离治疗窗。研究表明,环孢素血药浓度的个体间差异受患者生理、病理、遗传等因素的影响[1],尤其是与其体内过程相关的基因多态性,如CYP3A4*18B、CYP3A5*3、ABCB1(C1236T、G2677T/A、C3435T)、POR*28、PXR(C5705T、C39823T)和NFKB1-94 ins/del ATTG等。然而,目前如何根据基因型调整用药尚不明确,且针对环孢素注射液基因多态性的研究尚未见报道。因此,探究基因多态性对环孢素注射液血药浓度的影响,尝试建立以基因多态性为基础的个体化给药方案对优化临床用药具有一定的意义。
HTML
-
回顾性收集了某三甲医院泌尿外科收住的行肾移植术的患者144例。纳入标准:①患者年龄≥18岁;②住院期间使用环孢素注射液联合霉酚酸类药物和糖皮质激素。排除标准:①严重肝功能不全者(ALT或AST值≥3倍正常上限);②无法获取病案资料的患者;③同时服用显著影响环孢素血药浓度的药物,如氟康唑、伏立康唑、伊曲康唑及地尔硫䓬。
-
环孢素注射液连续多次给药后应用酶增强免疫分析法(EMIT)测定全血谷浓度,取稳态时的浓度作为最终的谷浓度(C0)。将剂量以体表面积进行校正,计算剂量校正谷浓度(C0/D′),即环孢素血药谷浓度/剂量×体表面积。
-
采用离心柱型全血DNA快速提取试剂盒提取全血样本中的基因组DNA,获得DNA样本浓度为10~60 ng/ml。基因分型采用Sequenom Mass ARRAY®SNP检测系统进行检测,由上海吉凯基因化学技术公司完成。
-
采用SPSS 24.0软件进行统计分析。计量资料以(
$\bar x \pm s$ )或Me(IQR)表示。采用独立样本t检验,比较单独使用环孢素注射液和联合使用环孢素注射液及糖皮质激素冲击治疗患者的C0及降低幅度的差异;采用χ2检验对各基因型分布进行Hardy-Weinberg平衡(HWE)检验;采用在线软件SHEsis(http://analysis.bio-x.cn/myAnalysis.php)分析各SNP位点间连锁不平衡(linkage disequilibrium,LD)状况;采用方差分析来计算各位点不同基因型患者间C0/D′的差异;采用Spearman相关性分析及多重线性回归分析建立基于基因多态性的个体化给药模型。
1.1. 研究对象
1.2. 全血谷浓度测定
1.3. 基因分型
1.4. 统计分析
-
入组患者共144例,其中男性109例,女性35例,患者基本信息见表1。
指标 统计描述 百分率(%) 年龄(岁) 37.5(15) 性别(男/女,例) 109/35 75.7/24.3 体重(kg) 59.46±10.47 体表面积(m2) 1.65(0.28) BMI(kg/ m2) 21.21±3.14 血红蛋白(g/L) 101.56±24.05 血细胞比容(%) 30.84±7.16 供体类型(尸体/亲属,例) 122/22 84.7/15.3 环孢素使用天数(d) 11(7) 是否激素冲击(是/否,例) 80/64 55.6/44.4 血肌酐是否降低(是/否,例) 112/32 77.7/22.3 注:数据以($\bar x \pm s$)或Me(IQR)表示 对患者环孢素注射液C0及用药后的血肌酐降低幅度进行统计分析。结果显示,单独使用环孢素注射液(单独用药组)后肌酐降低的患者比例为68.8%;联合使用环孢素注射液和糖皮质激素冲击治疗(联合用药组)后肌酐降低的患者比例为85.0%。单独用药组比联合用药组具有更高的C0〔(189.50±38.56)ng/ml vs(172.87±44.27)ng/ml〕,经独立样本T检验,两组患者C0的差异具有统计学意义(P<0.05)。然而,对两组患者的肌酐降低幅度进行Mann-Whitney U检验发现,单独用药组患者的肌酐降低幅度显著低于联合用药组〔19.27(22.84)% vs 45.25(50.38)%;P<0.01〕(见表2)。
用药方案 例数及比例(N/%) 肌酐降低例数及比例(N/%) C0(ng/ml) 肌酐降低幅度(%) 环孢素注射液 64(44.4) 44(68.8) 189.50±38.56 19.27(22.84) 环孢素注射液+糖皮质激素 80(55.6) 68(85.0) 172.87±44.27* 45.25(50.38)** 注:*P<0.05,**P<0.01,与单用环孢素注射液比较 -
入组患者的CYP3A4*18B、CYP3A5*3、ABCB1 C1236T、ABCB1 G2677T/A、ABCB1 C3435T、PXR C5705T、PXR C25385T、NFKB1 -94 ins /del ATTG及POR*28(仅64例患者)等9个SNPs的基因型频率分布及HWE遗传平衡吻合度计算见表3。9个SNPs均符合HWE(P>0.05),说明研究对象来自同一孟德尔群体,具有良好的群体代表性。
SNP(ID) 基因型频率(%) 等位基因频率(%) χ2 P 野生/野生 野生/突变 突变/突变 野生 突变 CYP3A4*18B GG(*1*1) GA(*1*18B) AA(*18B*18B) G A 0.046 0.830 rs2242480 80(55.6%) 54(37.5%) 10(6.9%) 74.3% 25.7% CYP3A5*3 AA(*1*1) AG(*1*3) GG(*3*3) A G 0.005 0.945 rs776746 11(7.6%) 57(39.6%) 76(52.8%) 27.4% 72.6% ABCB1 C1236T CC CT TT C T 0.985 0.321 rs1128503 14(9.7%) 70(48.6%) 60(41.7%) 34.0% 66.0% ABCB1G 2677T/A GG GT+GA TT+TA+AA G T+A 0.070 0.791 rs2032582 23(16.0%) 71(49.3%) 50(34.7%) 40.6% 59.4% ABCB1 C3435T CC CT TT C T 1.249 0.264 rs1045642 54(37.5%) 63(43.8%) 27(18.7%) 59.4% 40.6% PXR C5705T CC CT TT C T 2.918 0.088 rs3814055 87(60.4%) 45(31.3%) 12(8.3%) 76.0% 24.0% PXR C39823T CC CT TT C T 2.250 0.134 rs2276707 40(27.8%) 80(55.6%) 24(16.6%) 55.6% 44.4% NFKB1-94 ins /delATTG II ① ID ② DD ③ I D 0.036 0.849 rs28362491 45(31.3%) 72(50.0%) 27(18.7%) 56.3% 43.7% POR*28 CC CT TT C T 2.193 0.139 rs1057868 26(40.6%) 25(39.1%) 13(20.3%) 60.2% 39.8% 注:①. 指插入突变纯合子;②. 指插入突变杂合子;③. 指缺失突变纯合子。 -
各位点不同基因型患者之间使用环孢素注射液的C0/D′的差异见表4。在9个SNPs中,CYP3A4*18B基因多态性与环孢素注射液的C0/D′具有显著相关性,*1/*1基因型患者的C0/D′显著高于*18B/*18B基因型患者(P<0.05);CYP3A5*3、ABCB1 C1236T、ABCB1 G2677T/A、ABCB1 C3435T、PXR C5705T、PXR C39823T、NFKB1-94 ins/del ATTG及POR*28基因多态性均与肾移植患者环孢素注射液的C0/D′无显著相关性(P>0.05)。
位点(ID) 基因型 例数/占比[n,(%)] C0/D′[(ng/ml)/(mg/kg)] P CYP3A4*18B
rs2242480*1/*1 80(55.6) 1.52±0.42 0.044 *1/*18B 54(37.5) 1.42±0.42 *18B/*18B 10(6.9) 1.19±0.26* CYP3A5*3
rs776746*1/*1 11(7.6) 1.36±0.46 0.647 *1/*3 57(39.6) 1.45±0.37 *3/*3 76(52.8) 1.48±0.45 ABCB1 C1236T
rs1128503CC 14(9.70) 1.43±0.48 0.486 CT 70(48.6) 1.50±0.41 TT 60(41.7) 1.41±0.42 ABCB1 G2677T/A
rs2032582GG 23(16.0) 1.40±0.41 0.674 GA+GT 71(49.3) 1.49±0.40 AA+AT+TT 50(34.7) 1.44±0.46 ABCB1 C3435T
rs1045642CC 54(37.5) 1.46±0.41 0.596 CT 63(43.8) 1.43±0.43 TT 27(18.7) 1.53±0.43 PXR C5705T
rs3814055CC 87(60.4) 1.43±0.41 0.442 CT 45(31.3) 1.48±0.45 TT 12(8.30) 1.58±0.36 PXR C39823T
rs2276707CC 40(27.8) 1.55±0.49 0.262 CT 80(55.6) 1.43±0.40 TT 24(16.6) 1.38±0.35 NFKB1(-94 ins/del ATTG)
rs28362491II 45(31.3) 1.49±0.46 0.300 ID 72(50.0) 1.41±0.41 DD 27(19.7) 1.54±0.39 POR*28
rs1057868*1/*1 26(40.6) 1.63±0.45 0.491 *1/*28 25(39.1) 1.48±0.43 *28/*28 13(20.3) 1.54±0.38 注:*P<0.05,与*1/*1型比较。 -
参考单因素和多因素分析方法,将3个人口统计学指标(性别、年龄、体重)、3个临床指标(血红蛋白、血细胞比容、供体类型)和上述9个SNPs基因多态性定义为自变量,与环孢素注射液的C0/D′进行单因素相关分析。结果显示,在以上指标中,血红蛋白、血细胞比容和CYP3A4*18B基因多态性与环孢素注射液的C0/D′呈正相关,CYP3A4*18B与环孢素注射液的C0/D′呈负相关。其他12个指标与环孢素注射液C0/D′均没有显著相关性,详见表5。
指标 相关系数(r) 决定系数(R2) P 年龄 0.114 0.013 0.175 性别 −0.071 0.005 0.400 体重 0.140 0.020 0.094 血红蛋白 0.463 0.214 0.000* 血细胞比容 0.454 0.206 0.000* 供体类型 0.112 0.013 0.180 CYP3A4*18B −0.176 0.031 0.035 CYP3A5*3 0.043 0.002 0.610 ABCB1 C1236T −0.056 0.003 0.504 ABCB1 G2677T/A 0.006 0.000 0.943 ABCB1 C3435T 0.022 0.000 0.798 PXR C5705T 0.100 0.010 0.235 PXR C39823T −0.136 0.018 0.103 NFKB1(-94 ins/del ATTG) 0.003 0.000 0.971 POR*28 −0.130 0.017 0.305 对上述3个相关因素与环孢素注射液C0/D′进行的初步多重逐步回归分析,排除存在共线性问题的因素,得到最佳模型。在最佳回归模型中,血红蛋白和CYP3A4*18B基因多态性对环孢素注射液C0/D′均有统计学意义(P值分别为0.000和0.024)。根据最佳回归模型得到的回归方程即环孢素注射液C0/D′的预测算法,方程式如下:
Y=0.695+0.008X1−0.112X2,
式中因变量Y为环孢素注射液C0/D′,因此,应用环孢素注射液的肾移植患者环孢素注射液维持剂量预测模型为:
D′(mg/m2)=C/(0.695+0.008X1−0.112X2)
公式中:X1代表用药前患者的血红蛋白含量,X2代表CYP3A4*18B基因多态性;CYP3A4 *1/*1型患者X2=0,CYP3A4*1/*18B型患者X2=1,CYP3A4*18B/*18B型患者X2=2;C为临床TDM目标谷浓度值。
2.1. 环孢素注射液的临床疗效分析
2.2. 哈迪-温伯格平衡检验和基因型分布情况
2.3. 基因多态性与环孢素注射液血药浓度的相关性
2.4. 环孢素注射液个体化给药模型的建立
-
糖皮质激素冲击治疗是肾移植术后发生急性排斥反应的一线治疗方案。然而,由于糖皮质激素冲击治疗不良反应多且发生率高,对于临床上仅发生或疑似发生亚临床或临界排斥反应的患者可能并不是最佳用药。蔡治涛等[2]收录在《2012年中国器官移植大会论文汇编》中的研究指出以静脉环孢素为基础的免疫抑制治疗方案是一种安全、有效的治疗手段。为探究环孢素注射液在临床上的疗效,本研究对两种治疗方案(环孢素注射液单药vs环孢素注射液和糖皮质激素联合用药)对肾移植术后发生亚临床或临界排斥反应的临床疗效进行研究,结果发现,环孢素注射液单药组在降低患者肌酐水平方面的疗效不及环孢素注射液和糖皮质激素联合用药组〔肌酐降低比例:68.8% vs 85.0%;肌酐降低幅度:19.27(22.84)% vs 45.25(50.38)%;P<0.01〕,但单药组具有更高的稳态谷浓度C0〔(189.50±38.56)ng/ml vs (172.87±44.27)ng/ml〕。该研究结果表明对于肾移植术后发生亚临床或临界排斥反应的患者,单用环孢素注射液仍然具有较好的临床疗效,且血药浓度处于比较安全的剂量范围,可能是比较适合该类患者的治疗方案。
大量研究表明,遗传因素如编码药物转运体、代谢酶、作用靶点和核受体的基因多态性[3]是引起药物在人体内的处置和药物反应的个体性差异的主要原因,其中CYP3A4*18B、CYP3A5*3、ABCB1(C1236T、G2677T/A、C3435T)、POR*28、PXR(C5705T、C39823T)以及NFKB1-94 ins/del ATTG对环孢素药动学的影响较为重要。
CYP3A4和CYP3A5是环孢素的主要代谢酶,其编码基因的多态性可能影响酶的表达,进而影响环孢素的药动学。研究表明,CYP3A4*18B(或称CYP3A4*1G,82266G>A;rs2242480),是与环孢素的体内代谢相关的SNPs之一,在中国人群中突变频率为29.5%[4],该位点的突变可能会提高CYP3A4的活性[5],增加环孢素的代谢从而降低其血药浓度。Li等[6]研究发现,携带CYP3A4*18B/*18B基因型患者的环孢素血药浓度显著低于*1/*1及*1/*18B基因型患者,但*1/*1与*1/*18B基因型患者间差异无统计学意义。本研究结果显示,*1/*1基因型患者的环孢素注射液的C0/D′显著高于*18B/* 18B基因型患者(P<0.05),但*1/*1与*1/*18B基因型患者间差异无统计学意义。该结果与文献报道一致。CYP3A5*3(6986A>G;rs776746)是另一个与环孢素的体内代谢相关的SNP,在中国人群中的突变频率高达75.4%[7]。当*1突变为*3时可导致mRNA剪接发生改变和蛋白质截断,使CYP3A5酶活性降低或消失,减少环孢素经CYP3A5酶的代谢,从而使环孢素血药浓度升高。目前该位点基因多态性与环孢素血药浓度相关性的研究结论尚不统一[8-11]。本研究结果显示,CYP3A5*3基因多态性均与环孢素注射液的C0/D′无相关性。出现这一结果可能主要是环孢素由CYP3A酶系中的CYP3A4代谢,其对环孢素的清除率是CYP3A5的2.3倍[12]所导致。
P糖蛋白(P-gp)在环孢素的转运中发挥着重要作用,静脉给药可被肝脏P-gp将药物转移至胆道使药物胆汁排泄增加,从而使血药浓度降低。P-gp是多重耐药基因ABCB1编码的产物,因此ABCB1基因多态性可影响P-gp的表达从而影响环孢素的血药浓度。本研究结果显示,ABCB1 C1236T、ABCB1 C3435T和ABCB1 G2677T/A基因多态性与环孢素注射液的C0/D′无相关性,分析原因可能是由于P-gp主要位于小肠黏膜成熟上皮细胞的刷状缘上,只有小部分分布于肝细胞,因此,由ABCB1基因多态性导致的P-gp表达和活性的改变对环孢素注射液血药浓度的影响较小。
孕烷X受体(PXR)编码基因多态性很可能会影响PXR表达或功能,进而影响CYP3A酶及P-gp的表达,从而影响环孢素的药动学。研究报道[13-14],C5705T(rs3814055)、C39823T(rs2276707)与CYP3A4表型、活性和含量有关,且二者在中国人群中的突变频率分别是38.06%和78.95%[15]。但截至目前,PXR基因多态性是否与环孢素药动学具有相关性尚存在争议。本研究结果显示,PXR C5705T及C39823T基因多态性与环孢素注射液血药浓度无相关性。
NFKB1-94 ins/del ATTG中ATTG 4个碱基的缺失(deletion)引起启动子活性的降低,进而降低NF-κB的表达和功能,较少炎症反应的发生,从而减少对环孢素代谢的影响。Zhang等[10]研究发现,NFKB1 -94ATTG插入突变的纯合子个体(-94ATTG ins/ins)环孢素的C0/D'显著高于缺失突变(-94ATTG del/del)的个体〔(75.9±32.9)vs.(55.1±15.1)ng/ml per mg/kg,P=0.026〕。然而,本研究却发现缺失突变纯合子患者的C0/D′高于插入突变纯合子患者,但差异无统计学意义(P>0.05)。出现此结果的原因可能是该基因对环孢素的血药浓度的影响主要是通过受NF-κB的炎症反应而实现的,属于间接作用,所以可能对环孢素注射液的C0/D′影响较小,在其他主要影响因素的作用下导致缺失突变纯合子患者的C0/D′高于插入突变纯合子患者。
POR*28在中国人群中突变率为29.6%[16]。有研究报道,POR*28能够增加CYP3A的活性,从而增加环孢素经CYP3A的代谢使得其血药浓度降低。Elens等[17]分析了174例肾移植患者的POR*28的基因型及环孢素的部分药动学参数,结果发现,POR*28/*28患者的C0/D'较POR*1/*28和*1/*1患者低15.1%(CI 95%=224.8~24.2%;P=0.03),但POR*1/*28型和POR*1/*1型间无显著性差异。然而,本研究结果显示,POR*28/*28基因型患者环孢素的C0/D′与POR*1/*28和*1/*1基因型患者间并无显著性差异。
本研究所建立的给药剂量模型,只纳入血红蛋白和CYP3A4*18B基因多态性两个因素,经检验模型是有意义的(F=23.85,P<0.001),但该模型只能解释25.3%(R2=0.253)的个体差异,可能存在其他的对环孢素注射液C0/D′具有显著影响的因素,如药物相互作用(保肝药物、胃黏膜保护药物以及活血药物等)、患者术后内环境变化等,这些都可能影响环孢素注射液的体内药动学过程。这也是本研究存在的不足之处。
综上所述,本研究首次研究了基因多态性与环孢素注射液血药浓度的相关性并发现只有CYP3A4*18B基因多态性与环孢素注射液C0/D′呈显著相关,而其他8个SNPs基因多态性对环孢素注射液C0/D′无影响。但由于目前国内外关于环孢素注射液的相关研究几乎空白,现有参考资料较少,本研究作为初探,其结果仍需进一步进行大样本量的临床验证,对模型进行优化。








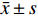
 DownLoad:
DownLoad: