-
光热疗法(photothermal therapy,PTT)是近年来新兴的肿瘤治疗技术,相比传统的手术治疗、化疗和放疗,具有更小的副作用[1-4]。PTT包含2个要素,即近红外光和光敏剂。近红外光具有较强的组织穿透能力[5-6],照射后肿瘤组织中的光敏剂可将光能转换为热能,当温度达到42 ℃以上时,可导致肿瘤细胞的凋亡或坏死[7]。
硫化铜(CuS)纳米粒是一种优良的光敏剂。相比广泛研究的金纳米粒,CuS纳米粒具有制备成本低的优点,特别是其具有稳定的光热效应。CuS纳米粒的近红外吸收主要源于Cu2+的d-d能级跃迁,因而其光热效应不受粒径、形状和生理环境的影响,可在肿瘤组织中维持稳定的光热效应[8]。CuS纳米粒具有良好的生物相容性,细胞毒性较低,但与其他多数无机或金属纳米粒同样存在体内发生蓄积的风险,长期使用的安全性未知。肾脏是处理纳米粒的主要器官之一,报道显示,直径小于6 nm的纳米粒可被肾脏滤过,从而使大部分纳米粒经尿液排出体外[9-10]。
因此,本文拟制备粒径小于6 nm的CuS纳米粒,通过深入分析各因素,获得最优的处方工艺,进一步通过光热曲线和细胞实验进行体外评价。
HTML
-
Litesizer500纳米粒度及Zeta电位分析仪(奥地利Anton Paar公司);JEOL 2010透射电子显微镜(日本电子株式会社);MDL-H-980-5W上转换发光用激光器(长春新产业光电技术有限公司);RX-300红外热成像仪(广东省东莞市不凡电子有限公司);NVMT SPARTAN夜视仪(梅越电子商务有限公司);DV215CD精密分析天平(美国Ohaus Discovery公司);HWS 26电热恒温水浴锅(上海一恒科学仪器有限公司);DF-101S集热式恒温加热磁力搅拌器(巩义市予华仪器有限责任公司);H1850R台式高速冷冻离心机(湖南湘仪实验室仪器开发有限公司);明澈-D24UV纯水/超纯水一体化系统(德国Merck Millipore公司)
-
二水合氯化铜(CuCl2·2H2O)、九水合硫化钠(Na2S·9H2O)(上海阿拉丁生化科技股份有限公司);聚维酮(polyvinylpyrrolidone,PVP,MW=10 000,默克生命科学有限公司);细胞增殖及毒性检测试剂盒(CCK-8,北京索莱宝生物技术有限公司);RPMI1640培养液,DMEM不完全高糖培养液,磷酸缓冲盐溶液(phosphate buffer saline,PBS)(江苏凯基生物技术有限公司);透析袋(MWCO 14000,美国Viskase公司);去离子水(实验室自制)。
1.1. 仪器
1.2. 材料
-
参考文献确定CuS纳米粒基本处方[11]:取100 μl CuCl2溶液(300 mmol/L)加入10 ml PVP溶液(100 mg/ml)中,再加入30 μl Na2S溶液(1 mol/L),室温磁力搅拌5 min后,转入90 ℃水浴继续磁力搅拌15 min,测定其粒径和多分散指数(polydispersity index,PDI)。
-
考察搅拌时间、水浴温度、水浴时间、PVP浓度、CuCl2与Na2S摩尔比(Cu:S)对CuS纳米粒粒径和PDI的影响。
表1结果显示,搅拌时间越长,水浴温度越低,Cu:S越小,则CuS纳米粒粒径呈减小趋势。水浴时间越长,PVP浓度越大,粒径显示了先增大后减小的趋势。综合各因素不同水平对应的纳米粒粒径,发现水浴温度、PVP浓度和Cu:S易产生较大的粒径波动,表明其可能是影响CuS纳米粒粒径的主要因素。另外,各因素不同水平对PDI的影响均不显著。因此,根据单因素考察结果,确定搅拌时间为5 min,水浴时间为15 min,以粒径为评价指标,采用星点设计-响应面法进一步优化水浴温度、PVP浓度、Cu:S三个因素。
因子 水平 粒子大小 (nm) 多分散指数 搅拌时间 (min) 3 18.13±5.42 0.27±0.01 5 16.12±1.29 0.27±0.01 7 15.40±1.86 0.26±0.01 水浴温度 (℃) 80 14.61±4.35 0.27±0.01 90 16.12±1.29 0.27±0.01 100 22.85±1.71 0.26±0.01 水浴时间 (min) 10 18.12±6.31 0.25±0.02 15 16.12±1.29 0.27±0.01 20 21.30±5.71 0.24±0.01 PVP浓度 (mg/ml) 80 25.56±4.40 0.27±0.01 100 16.12±1.29 0.27±0.01 120 20.72±3.38 0.25±0.02 Cu:S 3:1 15.08±2.92 0.26±0.01 2:1 20.25±1.16 0.27±0.01 1:1 16.12±1.29 0.27±0.01 1:2 11.34±3.08 0.25±0.02 1:3 11.27±2.88 0.25±0.02 -
在单因素考察的基础上,采用中心复合响应面设计(central composite design, CCD),选择上述筛选出的三个因素:水浴温度(X1)、PVP浓度(X2)、Cu:S(X3)为考察因素,粒径(Y1)为评价指标,进行三因素五水平的星点设计。
-
星点实验设计的因素、水平及代码值如表2所示,实验设计与结果如表3所示。
因子 水平 −1.682 −1 0 +1 +1.682 X1 (℃) 60.00 70 85 100 110.20 X2 (mg/ml) 66.36 80 100 120 133.64 X3 0.25 0.33 1.67 3 4 序号 X1 (℃) X2 (mg/ml) X3 Y1 (nm) 1 −1 −1 −1 13.04 2 +1 −1 −1 14.23 3 −1 +1 −1 14.48 4 +1 +1 −1 13.39 5 −1 −1 +1 24.37 6 +1 −1 +1 27.30 7 −1 +1 +1 18.62 8 +1 +1 +1 20.73 9 −1.682 0 0 18.87 10 +1.682 0 0 15.62 11 0 −1.682 0 24.01 12 0 +1.682 0 17.06 13 0 0 −1.682 13.92 14 0 0 +1.682 15.11 15 0 0 0 18.81 16 0 0 0 16.84 17 0 0 0 16.14 18 0 0 0 17.89 19 0 0 0 16.20 20 0 0 0 16.30 -
采用Design expert 8.0.6,将各影响因素(X1、X2、X3)和评价指标(Y1)进行二次多项式模型拟合,方程如下:
Y1=4.15−0.0086X1−0.18X2+0.42X3−0.045X1X2+0.064X1X3−0.17X2X3+0.043X12+0.17X22−0.22X32
此时二项式模型达显著水平(P=0.006 5),r=0.9129,说明该方程与实际情况拟合度良好。
-
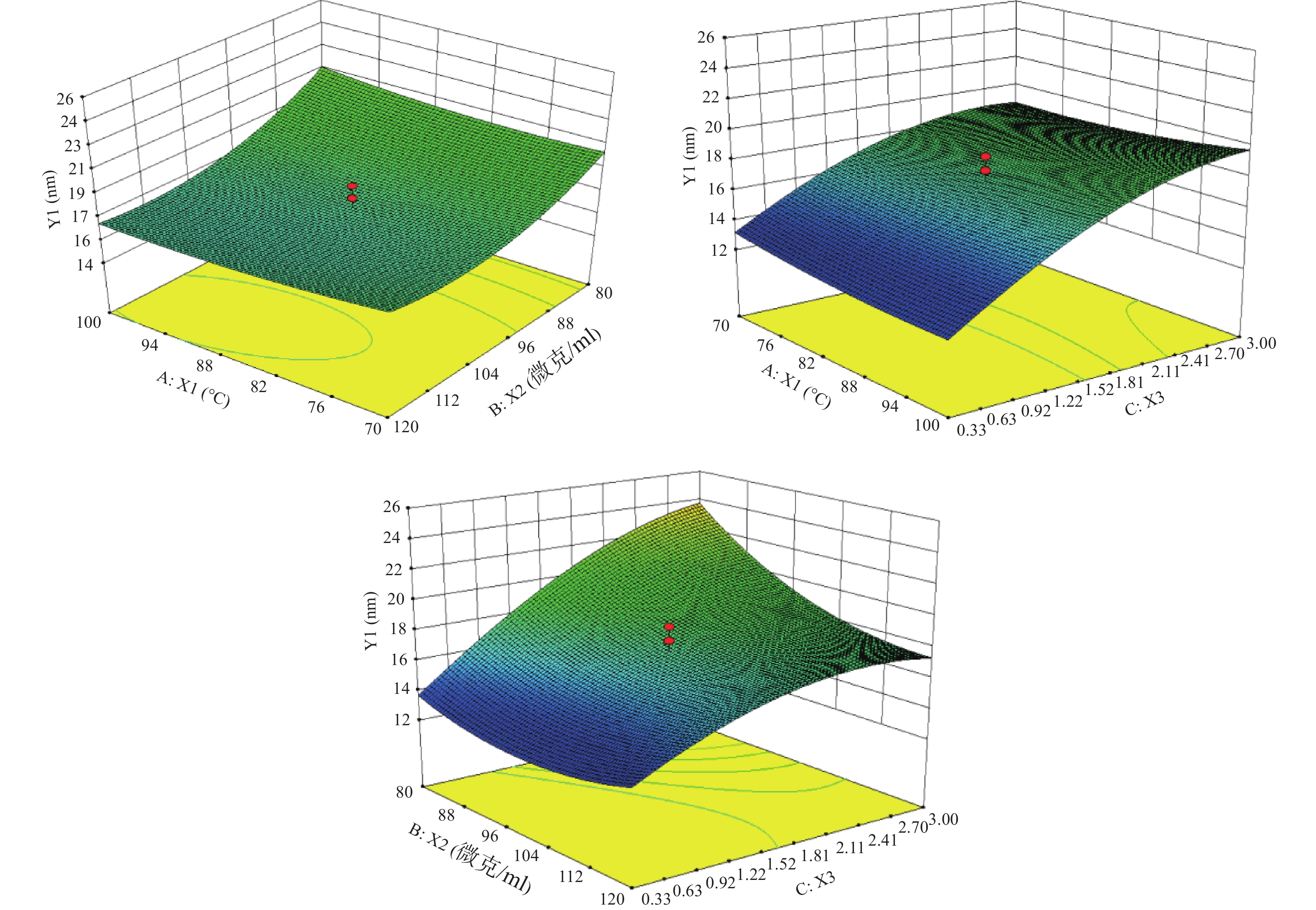
根据表3结果,绘制水浴温度(X1)、PVP浓度(X2)及Cu:S(X3)对粒径(Y1)的响应面。
根据图1的数据进行分析,确定最优参数为:水浴温度100 ℃,PVP浓度为100 mg/ml,Cu∶S为1∶3。按照最优处方工艺平行制备3份样品,粒径分别为9.37、12.39、9.84 nm,平均值为(10.53±1.63)nm,预测值为10.86 nm,根据公式:偏差=(预测值−实际值)/预测值×100%,得出实际值与预测值的偏差为3.04%,说明建立的数学模型较为可靠,预测性良好。
-
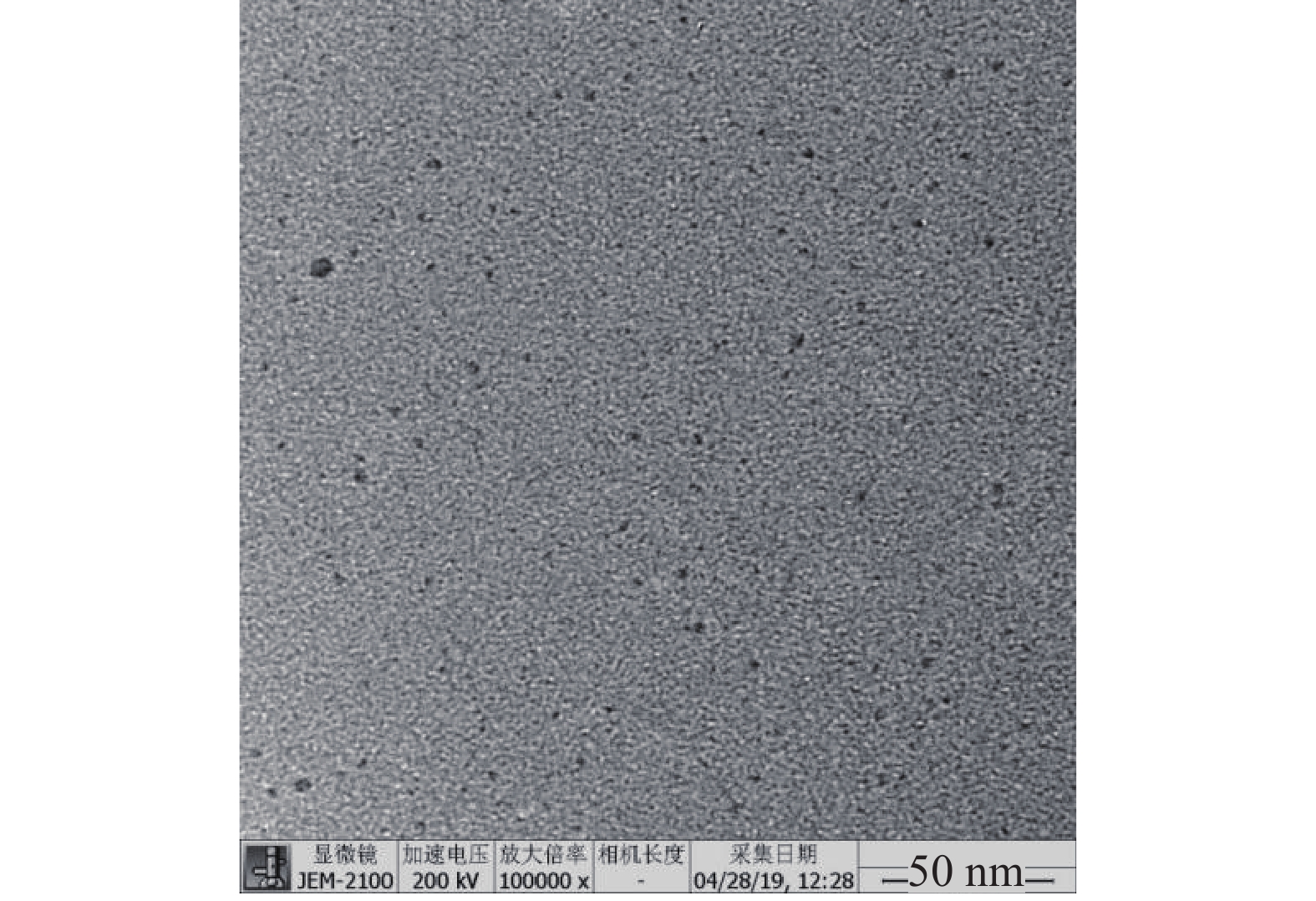
取CuS纳米粒适量,加去离子水稀释10倍,取5 μl稀释后的CuS纳米粒,滴于缚有碳支持膜的铜网上,铜网下铺滤纸,用于吸去多余的液体。自然晾干后,用透射电子显微镜(transmission electron microscope, TEM)观察纳米粒形态。图2结果显示,CuS纳米粒形态圆整,分散性良好,经Image J软件测量,平均粒径为(3.10±0.81)nm。
-
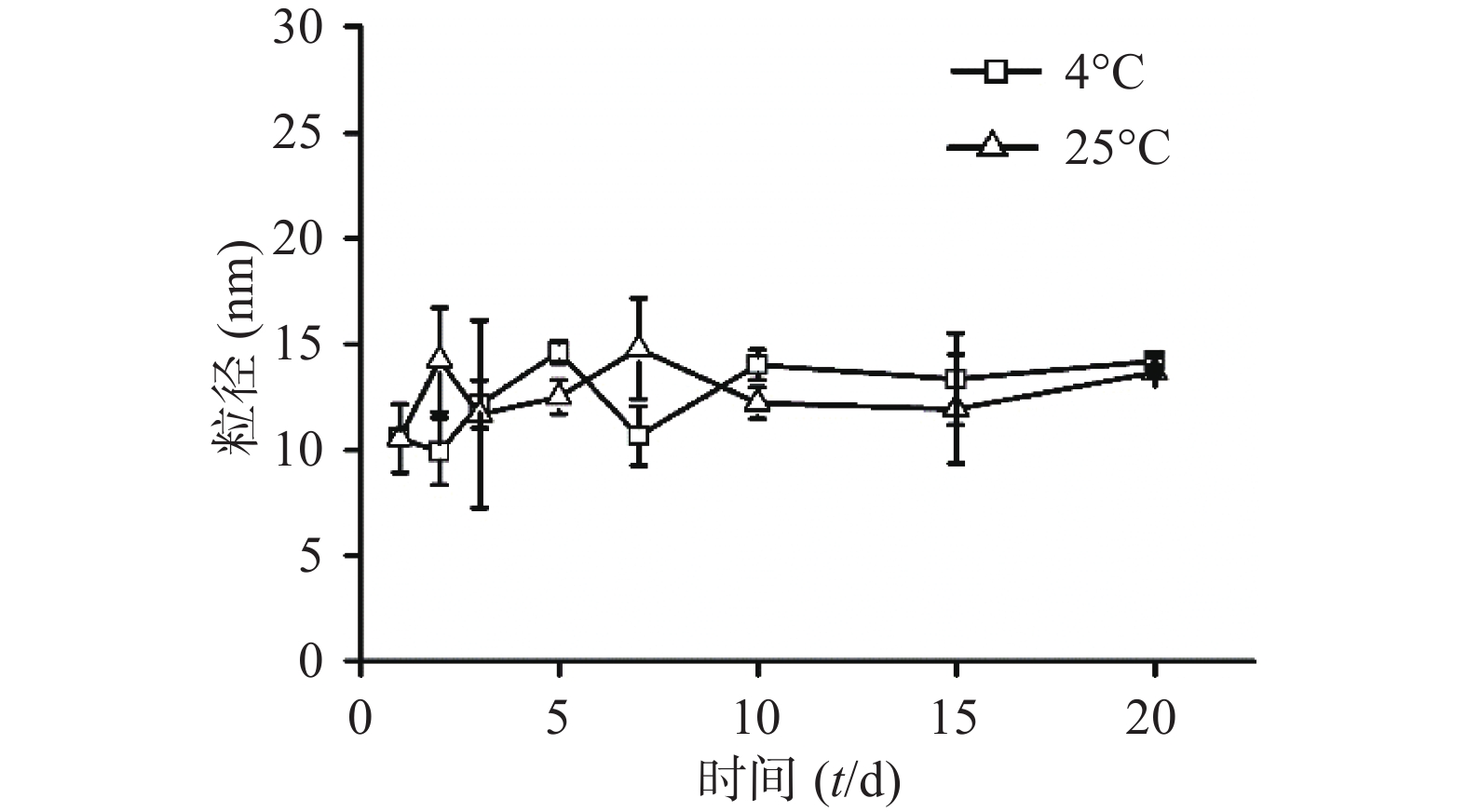
将CuS纳米粒分别放置在4 ℃与25 ℃条件下,定时测定粒径。经统计发现,20 d内CuS纳米粒的粒径变化没有明显差异,说明所制的CuS纳米粒具有良好的粒径稳定性(图3)。
-
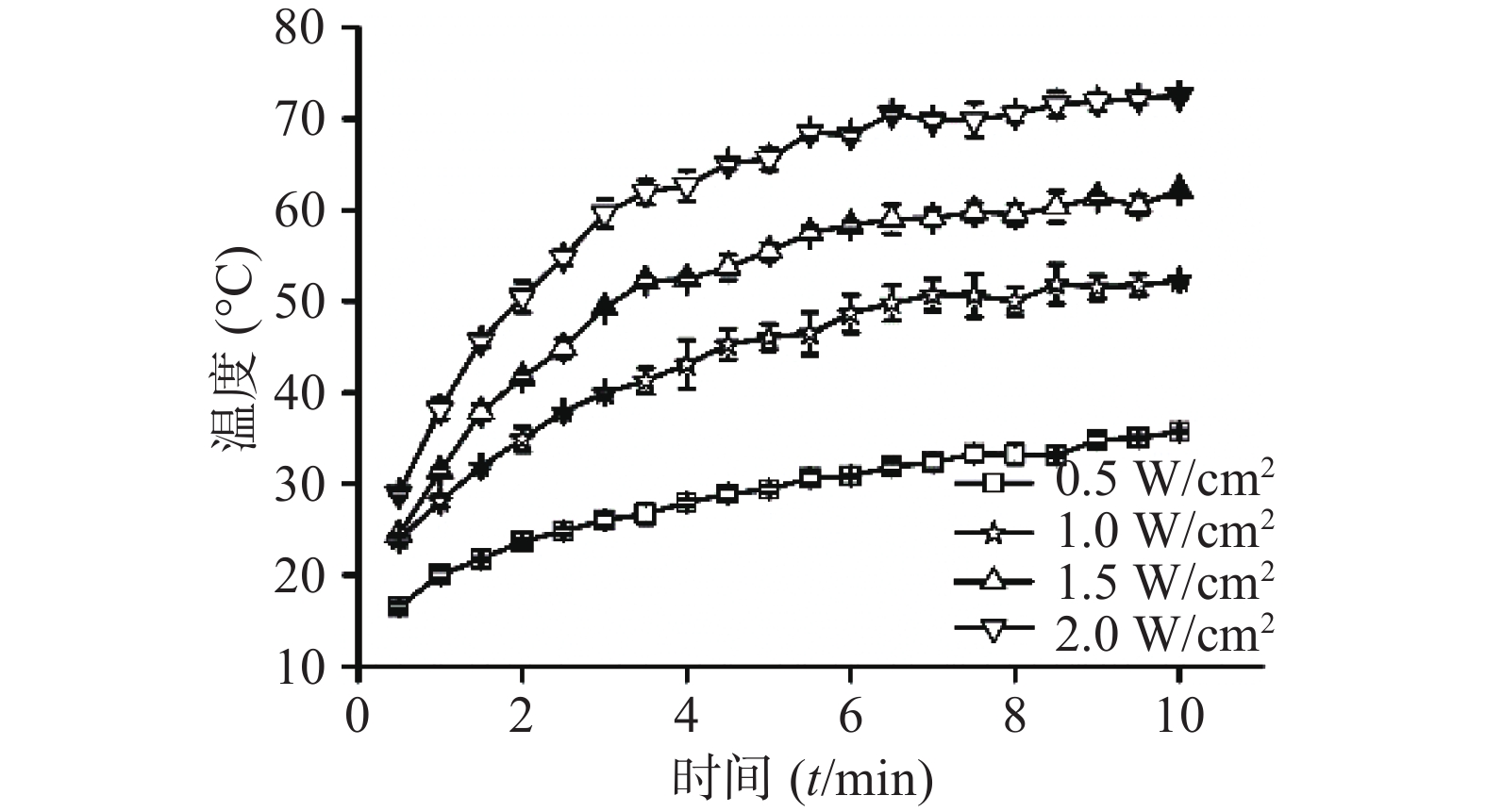
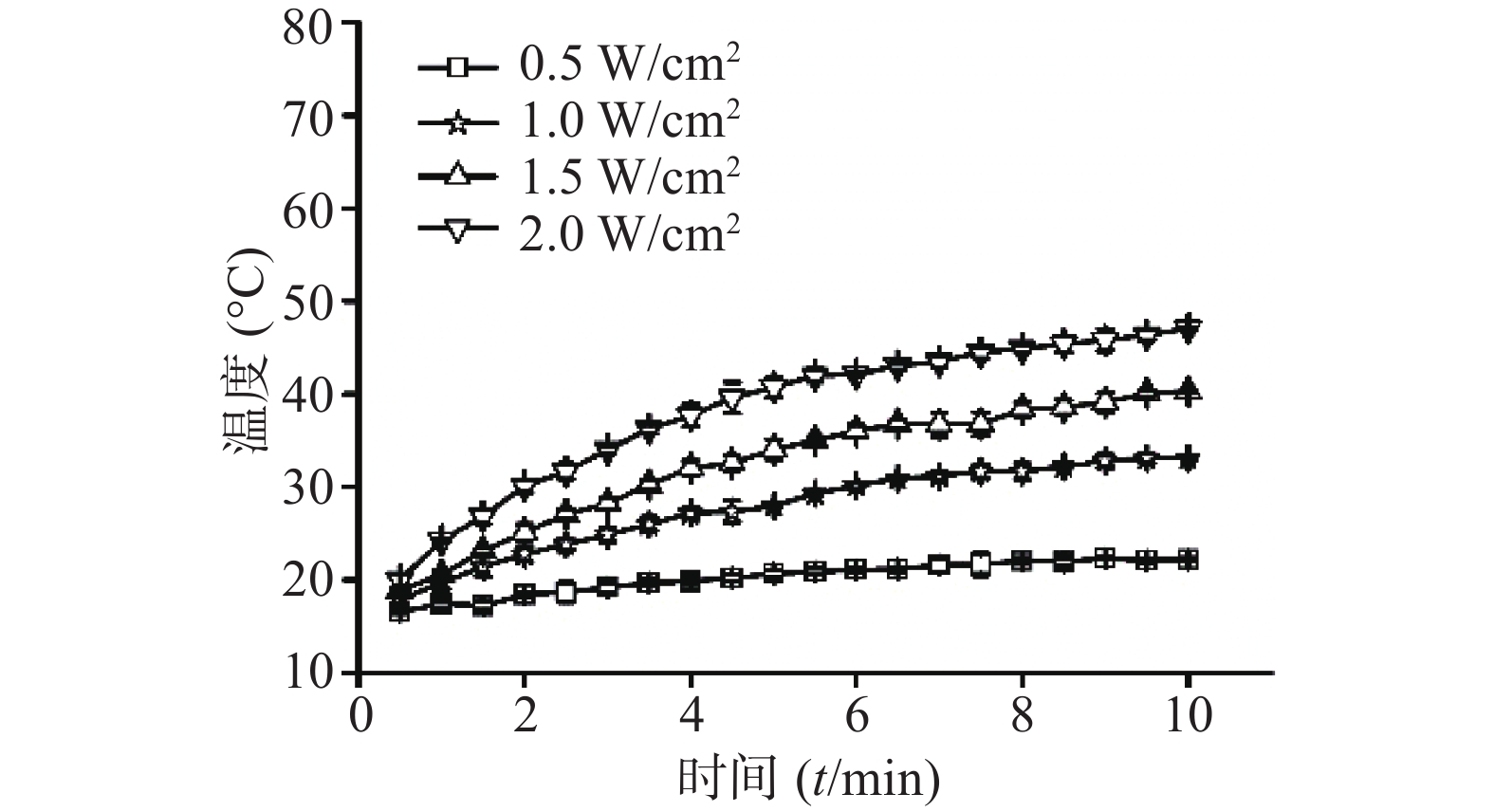
⑴近红外光功率密度:考察CuS纳米粒浓度为50 μg /ml条件下,不同近红外光功率密度(0.5 、1、1.5、2 W/cm2)对CuS纳米粒光热效应的影响。采用980 nm的近红外激光器,红外热成像仪每隔30 s记录温度,记录10 min,平行测量3组,绘制升温曲线,同时,以水在相同条件下做对照。随着功率密度增大,CuS纳米粒升温加快(图4),而在相同条件下,去离子水的升温速率显著低于CuS纳米粒(图5)。
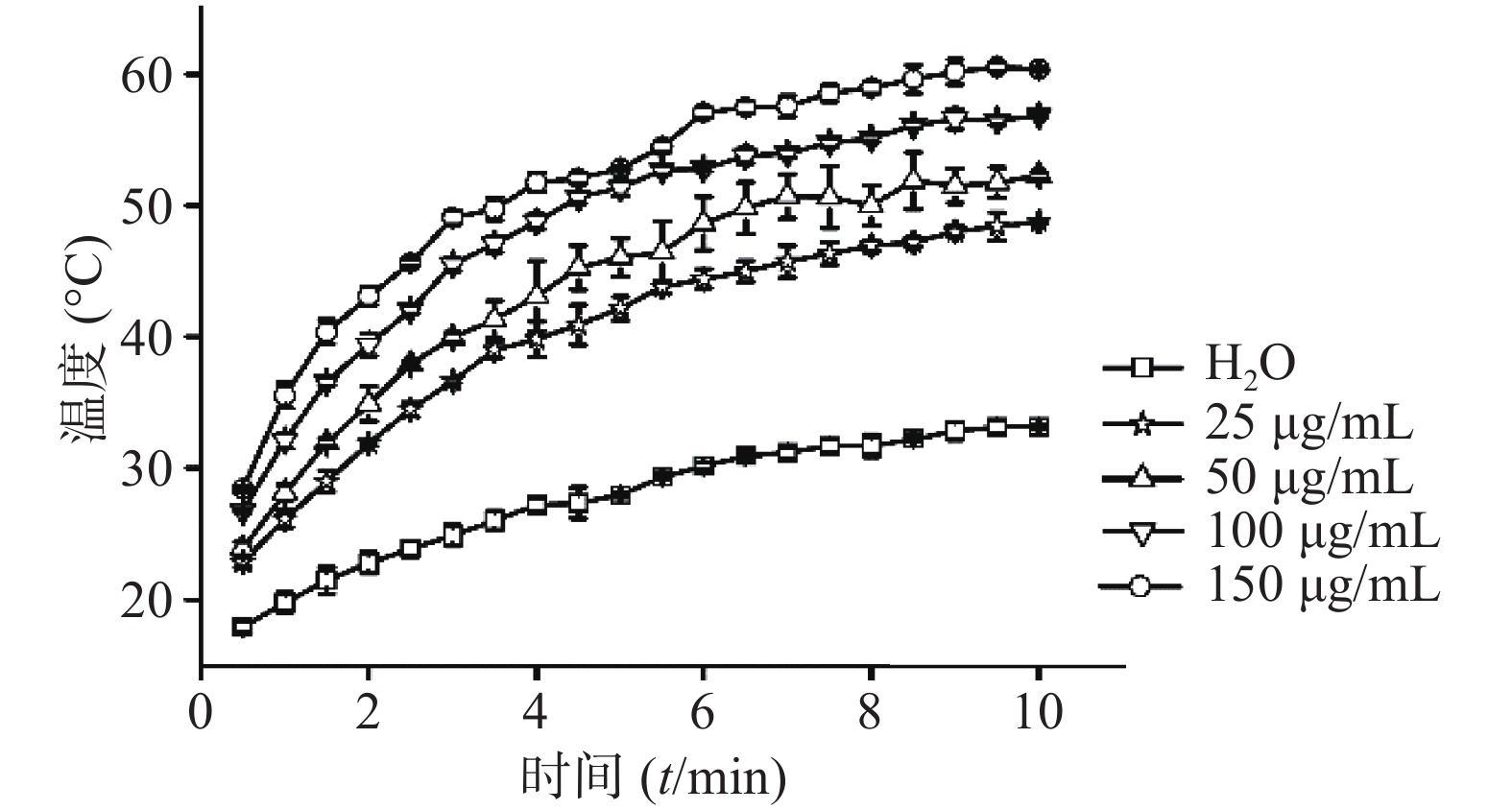
⑵CuS纳米粒浓度:考察在1 W/cm2功率密度下,CuS纳米粒不同浓度(25 、50 、150 、200 μg/ml)对其光热效应的影响,并以去离子水为空白对照。采用980 nm的近红外激光器,红外热成像仪每隔30 s记录温度,记录10 min,平行3组,绘制升温曲线。结果显示,在相同功率密度条件下,CuS纳米粒浓度越大,升温速率越大(图6)。
-
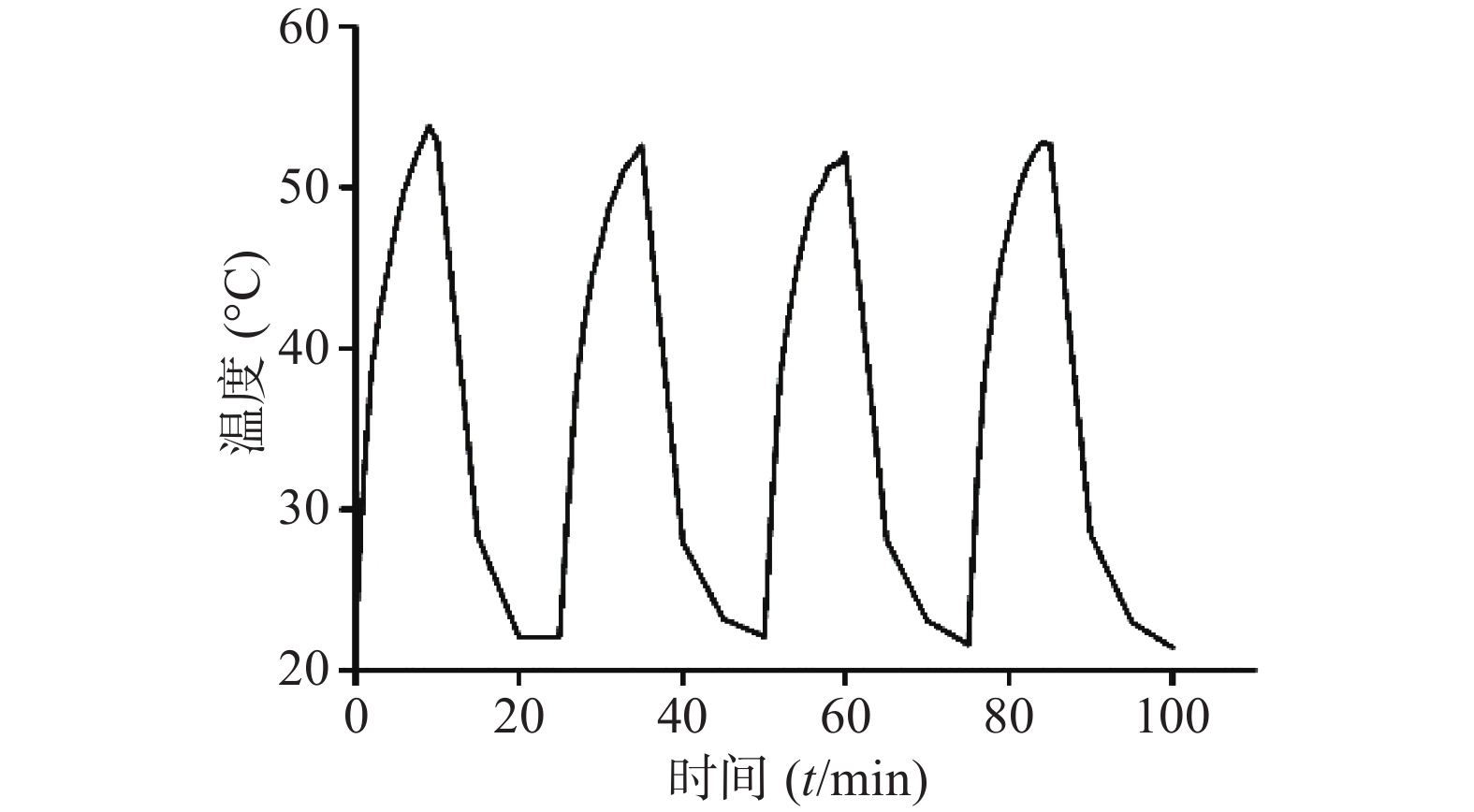
采用980 nm近红外激光器,选取CuS浓度为50 μg /ml,激光功率密度为1 W/cm2,将激光器“开启-关闭”4个循环,即照射10 min后停止,温度恢复至室温后再次开启照射。如图7所示,CuS纳米粒经近红外光照射4个循环的温度变化曲线基本一致,即反复照射不影响CuS纳米粒的光热效应,表明其具有良好的光热稳定性。
-
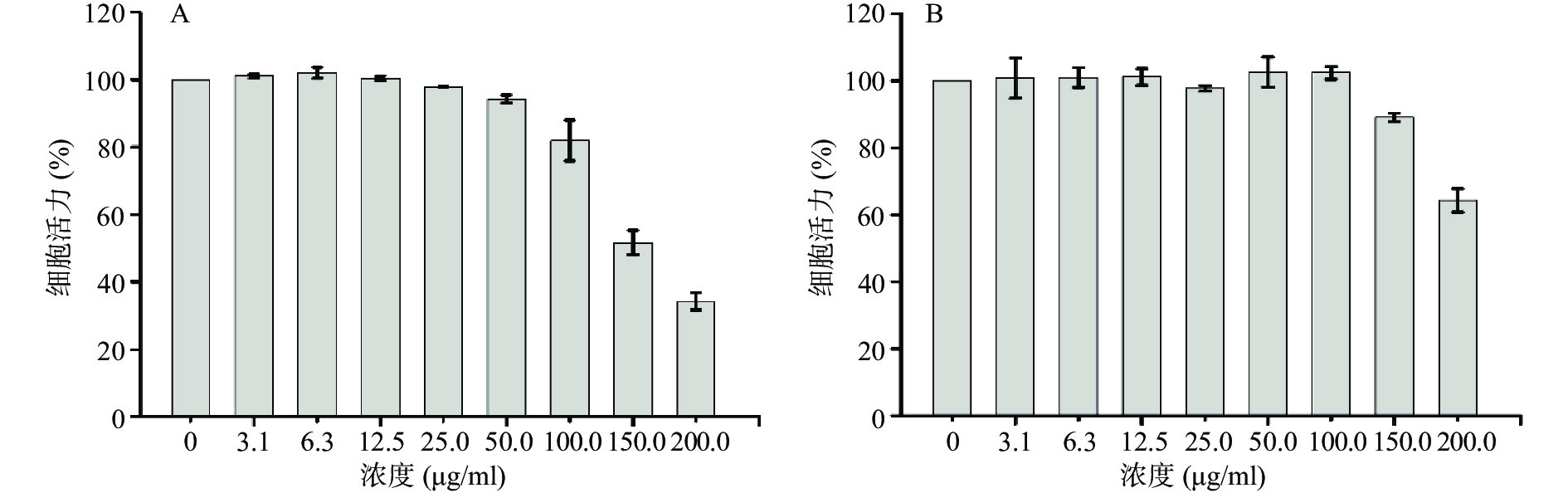
考察CuS纳米粒对正常人肾小管上皮细胞(HK2)以及乳腺癌细胞(4T1)的毒性。实验前,取适量CuS纳米粒放入透析袋(MWCO=14000)中,透析24 h以除去杂质。取对数生长期细胞,调整HK2细胞浓度为1×105 cells/ml,4T1细胞浓度为5×104 cells/ml,将100 μl/孔的细胞悬液接种于96孔细胞培养板,置于37 ℃,5% CO2培养箱中。孵育24 h后,每孔加入10 μl不同浓度的CuS纳米粒,使终浓度分别为3.1、6.3、12.5、25、50、100、150及200 μg/ml,继续培养24 h后,去除96孔板中培养基,加PBS洗涤2次(以排除Cu2+对CCK-8试剂的影响),每孔加入100 μl培养基及10 μl CCK-8试剂,于培养箱中孵育4 h后,用酶标仪测定450 nm处的吸光度值(A)。参照公式:
细胞活力(%)=(A加药−A空白)/(A0加药−A空白)×100%,
计算细胞活力百分率,其中A加药指具有细胞、CCK-8溶液和药物溶液的孔的吸光度,A空白指具有培养基和CCK-8溶液而没有细胞的孔的吸光度,A0加药指具有细胞、CCK-8溶液而没有药物溶液的孔的吸光度。
结果显示(图8),CuS纳米粒分别在100 μg/ml及150 μg/ml浓度范围内,对4T1乳腺癌细胞以及HK2肾细胞均无显著的细胞毒性(细胞活力大于80%),表明CuS纳米粒具备良好的生物相容性。
-
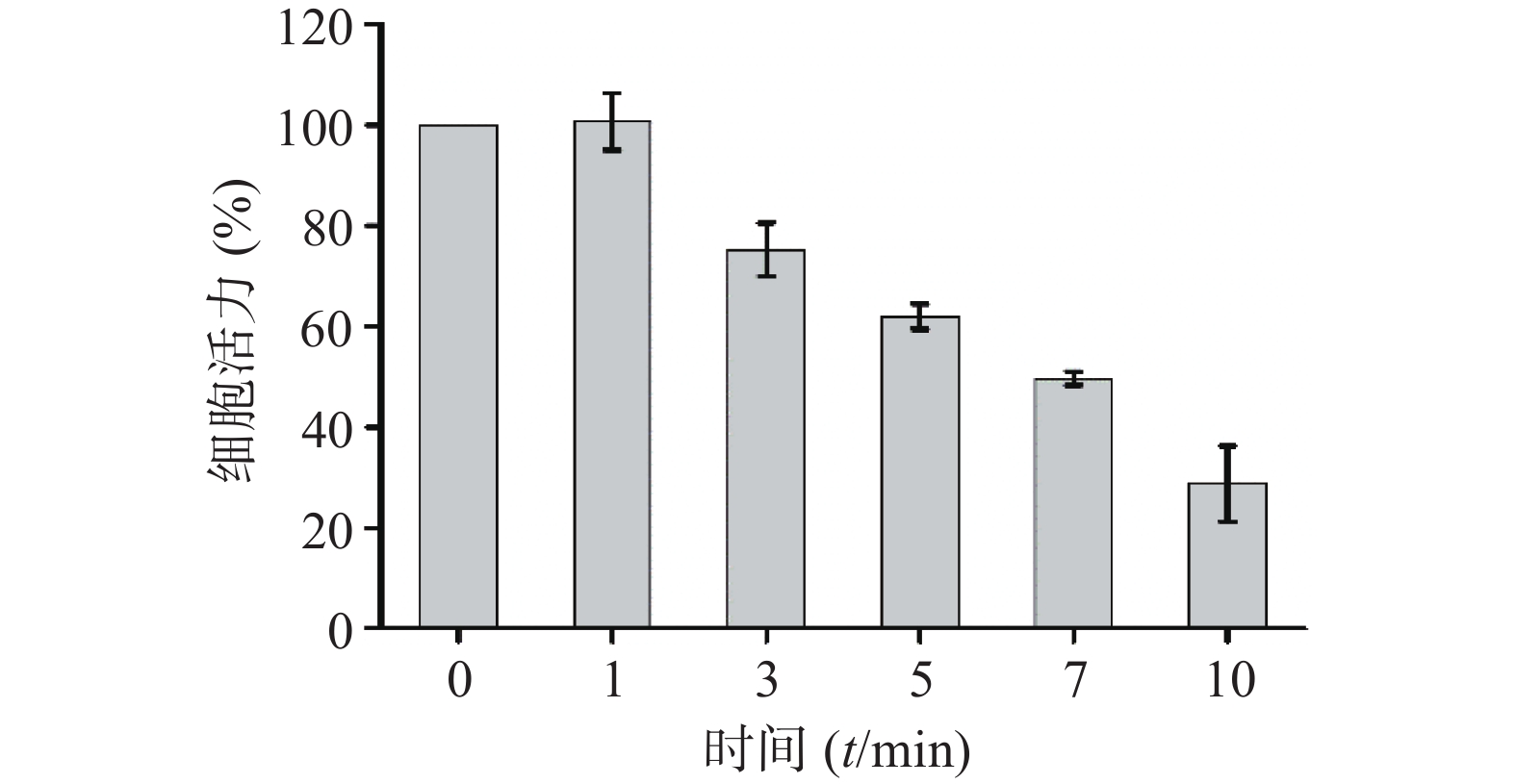
考察CuS纳米粒的光热效应对4T1细胞的杀伤作用。取对数生长期细胞,调整4T1细胞浓度为5×104 cells/ml,将200 μl/孔细胞悬液接种于96孔细胞培养板,置于37 ℃,5% CO2培养箱中培养24 h后,去除全部培养基,加入200 μl含CuS纳米粒的培养基(CuS纳米粒的浓度为50 μg /ml)。采用1 W/cm2的近红外光,照射不同时间(0、1、3、5、7、10 min),随后采用CCK-8试剂测定细胞活力百分率,参照“2.5.1”项下公式计算。结果显示(图9),近红外光照射1 min对4T1细胞的活力无明显影响,继续延长照射时间,细胞活力呈显著的降低趋势,照射10 min后的细胞活力仅为28.76 %。
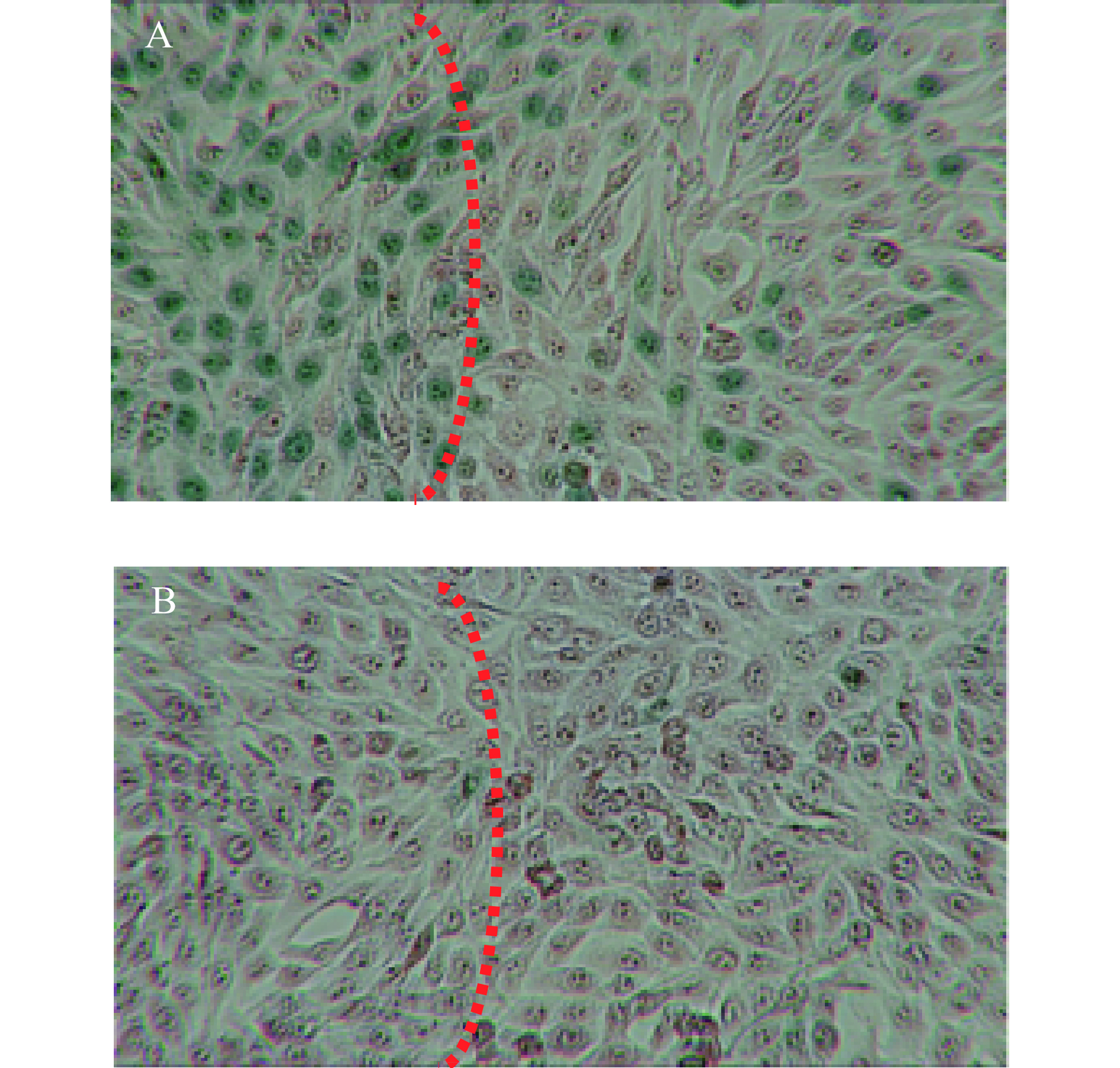
采用与上述相同的方法,改为6孔板,经980 nm近红外光(1 W/cm2)照射10 min后,加入0.2 %台盼蓝对死细胞进行染色(2 min)。去除染液并用PBS清洗2遍后,在倒置相差显微镜下进行观察。如图10所示,激光照射区域,细胞被染为蓝绿色,且显示了明显的边界,而对照组不加CuS纳米粒,仅给予近红外光照射,只有个别零散的细胞着色。图9与图10结果均表明CuS纳米粒对肿瘤细胞具有显著的光热杀伤效果。
2.1. 硫化铜纳米粒的制备
2.2. 单因素考察
2.3. 星点设计-响应面法优化CuS纳米粒处方工艺
2.3.1. 星点设计结果
2.3.2. 模型拟合
2.3.3. 响应面优化及预测性评价
2.4. CuS纳米粒的体外评价
2.4.1. 纳米粒形态
2.4.2. 粒径稳定性
2.4.3. 光热效应
2.4.4. 光热稳定性
2.5. 体外细胞实验
2.5.1. 细胞毒性实验
2.5.2. 光热效应对肿瘤细胞的杀伤作用
-
目前光热治疗作为新型的肿瘤治疗技术,寻找合适的光敏剂是亟待解决的重要问题。光热稳定、价格低廉等是CuS纳米粒的主要优点,但目前文献所报道的CuS纳米粒粒径多在10 nm以上[12-13],可能引起CuS纳米粒在体内的蓄积,将限制其在临床上的使用,而减小粒径可以有效解决这个问题。本研究经单因素考察和星点设计-响应面优化法,得到CuS纳米粒的最优处方工艺,成功制备实际粒径为(3.10±0.81)nm的CuS纳米粒,且制备方法简单,易于工业化生产。该处方中以PVP作为保护剂,使CuS纳米粒具有良好的粒径稳定性,分散度高,不易发生聚集沉淀,单因素考察分析也可发现PVP浓度对CuS纳米粒粒径具有较大影响,PVP浓度低,CuS纳米粒容易发生聚集,粒径增大;浓度太高,保护层过厚,也会引起CuS纳米粒粒径增大,因此选择合适的PVP浓度,是控制CuS纳米粒粒径关键因素之一。
由于CuS纳米粒在近红外区域(700~1 400)nm具有强吸收,使其在近红外光照射下,具有较强的光热效应,所优选的CuS纳米粒在1 W/cm2的激光功率密度下,照射4 min,温度即可达到42 ℃以上,可引起肿瘤细胞的凋亡或坏死[14]。因CuS纳米粒的光热转换性能来源于Cu2+的d-d能级跃迁,不易受外界环境的影响,所优选的CuS纳米粒具有良好的光热稳定性。本实验所优选的CuS纳米粒生物相容性好,且对肿瘤细胞具有显著的光热杀伤作用,这与多数文献报道相一致[15-16]。
综上,本研究所制备的CuS纳米粒粒径有望解决CuS纳米粒体内蓄积的问题,使其更好地应用于肿瘤光热治疗。本课题将进一步研究所制硫化铜纳米粒在体内的代谢情况以及对肿瘤组织的光热杀伤效果。








 DownLoad:
DownLoad: