-
脑中风是由于脑部血管突然破裂或因血管阻塞导致血液不能流入大脑而引起脑组织损伤的一类疾病[1]。大多数患者在治疗后会遗留不同程度的神经功能缺损,比如偏瘫、嘴歪眼斜、智力障碍、语言认知功能丧失等[2]。因而开发神经恢复剂,修复损伤神经功能尤为重要。修复受损神经功能的途径之一是促进被破坏或受损害的神经重塑[3]。神经重塑通过诱导神经元分化,促进突起向外生长,与其他神经元建立连接,重构皮层,从而修复损伤的神经功能[4]。
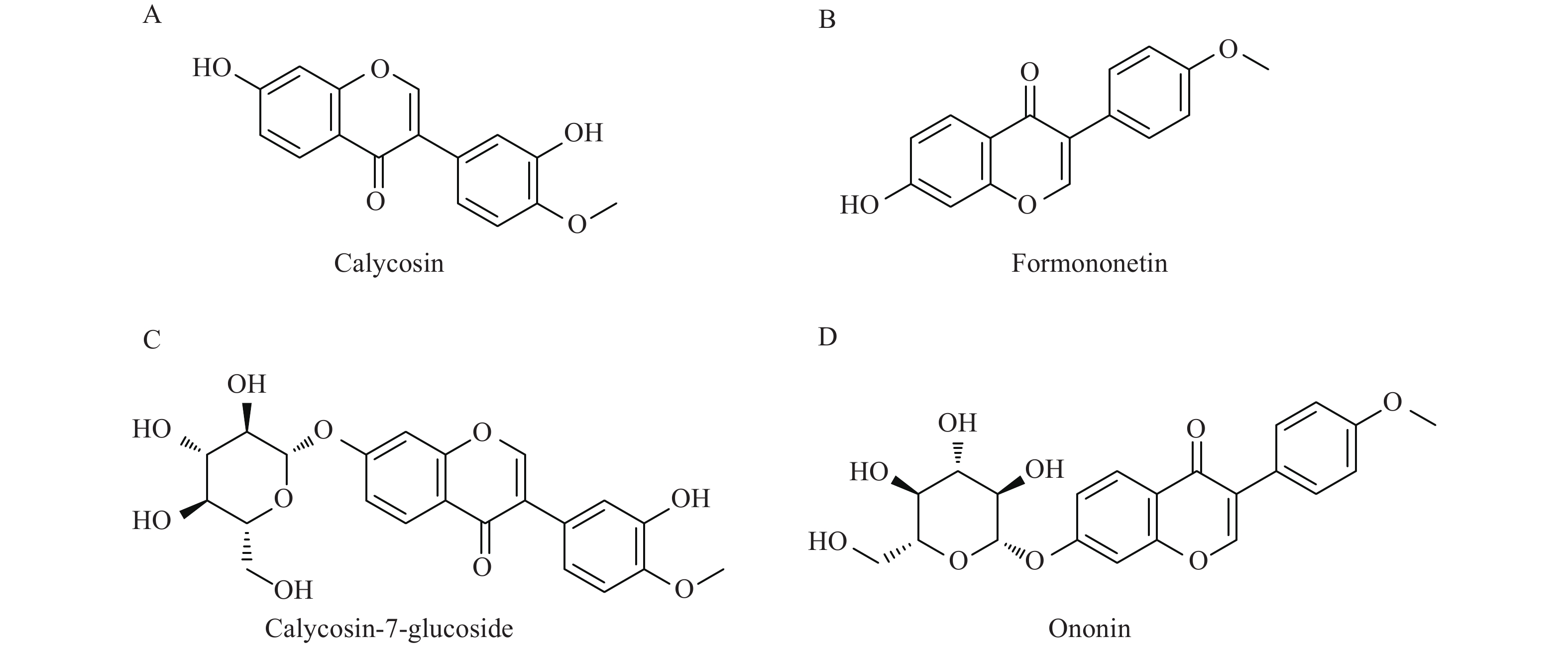
黄芪异黄酮类化合物主要包括毛蕊异黄酮、刺芒柄花素、毛蕊异黄酮苷、芒柄花苷,结构如图1所示[5]。毛蕊异黄酮、刺芒柄花素、毛蕊异黄酮苷、芒柄花苷对急性脑缺血具有神经保护作用,对缺血后脑损伤神经功能的恢复作用未见报道[6-8]。
PC 12细胞是来源于成年大鼠肾上腺髓质嗜铬细胞瘤的细胞系,培养PC 12细胞可动态观察其神经分化的过程[9],因此可将PC 12细胞作为筛选促神经分化、恢复损伤神经功能药物的理想模型。本项研究工作使用PC 12细胞,观察毛蕊异黄酮、刺芒柄花素、毛蕊异黄酮苷、芒柄花苷是否具有诱导细胞神经分化的能力。神经生长因子(nerve growth factor, NGF)具有明确的促进神经元分化作用[10],在本研究中用作阳性对照。
HTML
-
毛蕊异黄酮、毛蕊异黄酮苷、芒柄花苷(纯度≥98%,上海源叶生物科技有限公司);刺芒柄花素(纯度≥98%,成都普瑞法科技开发有限公司);二甲基亚砜(dimethyl sulfoxide,DMSO,Sigma公司);多聚-L-赖氨酸(Biosharp公司);胎牛血清(ScienCell公司);特级马血清(索莱宝公司);丙酮酸钠和RPMI 1640培养基(Gibco公司);磷酸盐缓冲液(博光公司);4',6-二脒基-2-苯基吲哚(DAPI)和Triton X-100(碧云天);4%多聚甲醛(武汉赛维尔生物科技有限公司);重组大鼠β-神经生长因子(NGF,R & D公司);β微管蛋白(β III-tubulin,Abcam公司);Alexa Fluor TM 568兔抗小鼠IgG(H+L)(Invitrogen公司)。
6孔板培养皿和10 ml培养皿(美国Corning公司);倒置荧光显微镜 (DMI3000 B,德国Leica公司);精密电子天平(美国Ohaus公司)。
-
PC 12细胞购自上海中国科学院细胞库,经复苏后,加入含10%马血清、5%胎牛血清、1%丙酮酸钠、100 U/ml青霉素和100 μg/ml链霉素的RPMI 1640培养基,放置于培养箱(37 ℃,95%空气,5%CO2)中传代培养,每2 d传代1次。
-
将PC 12细胞以5×104 /ml的密度涂覆在多聚-L-赖氨酸包被过的6孔板中,放置于培养箱中孵育过夜。24 h后,换分化培养基(含1%马血清、1%胎牛血清、1%丙酮酸钠、100 U/ml青霉素和100 μg/ml链霉素),加不同浓度NGF(0.3~100.0 ng/ml)、毛蕊异黄酮、刺芒柄花素、毛蕊异黄酮苷、芒柄花苷(0.01~10.00 μmol/L)继续培养。每天更换一次上述化合物和分化培养基,连续3 d。培养5 d后在显微镜下观察各组PC 12细胞突起向外生长的情况。将长度超过一个细胞体的神经突记为阳性细胞[11]。在每孔中随机选择5个视野,分别计算神经突细胞的百分比,然后计算平均值。
-
PC 12细胞处理方式同“1.3”项,培养5 d后用免疫荧光染色方法检测β III-tubulin蛋白的表达。各组PC 12细胞经4%多聚甲醛固定20 min、Triton X-100透化15 min、5%脱脂牛奶封闭1 h、一抗β III-tubulin(1:100)4 ℃孵育过夜、二抗(1:500)室温孵育1 h、DAPI室温染色20 min后在荧光显微镜下观察β III-tubulin和DAPI的表达情况。在每孔中随机选择5个视野,分别统计β III-tubulin表达的阳性细胞数目,然后计算平均值。
-
采用Graphpad软件分析,数据采用(均值±标准误)表示,组间数据采用方差分析,以P<0.05表示有显著性差异,P<0.01表示有极显著性差异。
1.1. 试剂与仪器
1.2. PC 12细胞培养
1.3. NGF及黄芪异黄酮类化合物对PC 12细胞突起向外生长的影响
1.4. NGF及黄芪异黄酮类化合物对PC 12细胞β III-tubulin蛋白表达的影响
1.5. 数据分析
-
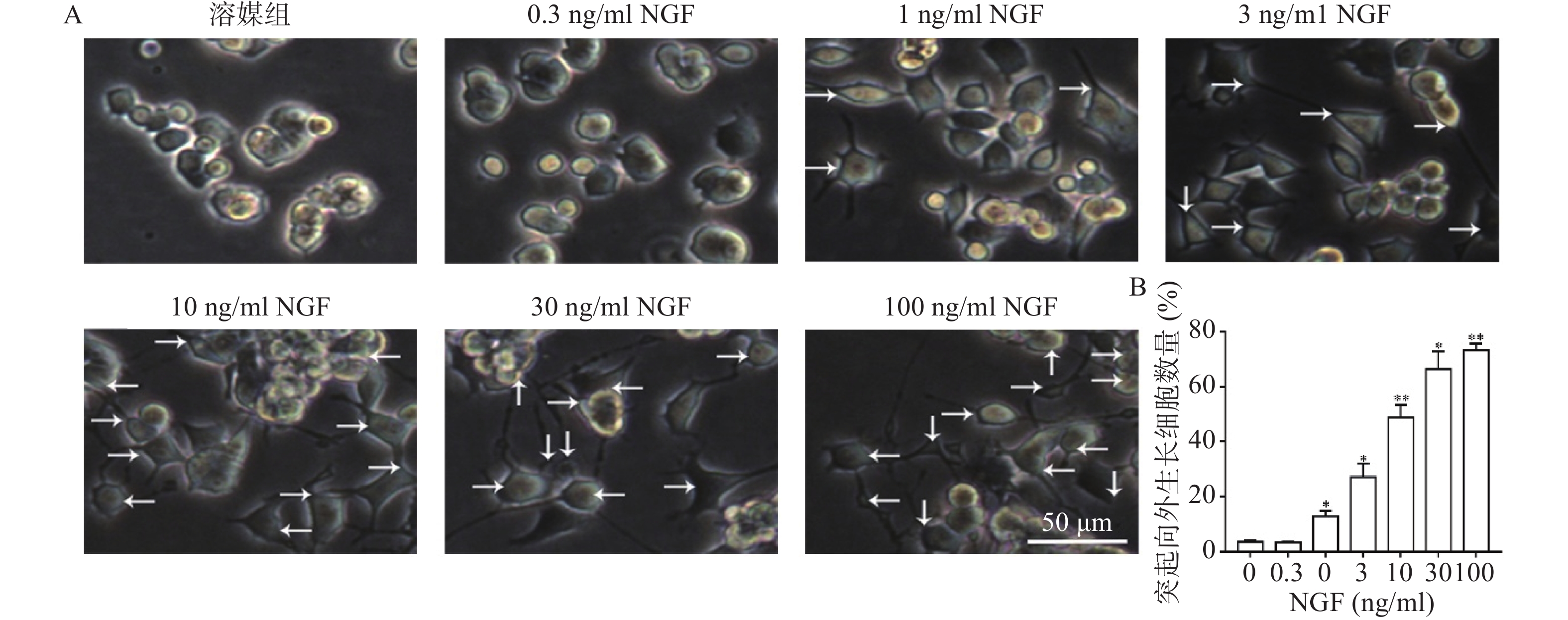
如图2所示,与溶媒组相比,0.3 ng/ml NGF对PC 12细胞突起向外生长无促进作用。与溶媒组相比,NGF(1~100 ng/ml)显著促进PC 12细胞突起向外生长,且具有浓度依赖性。
-
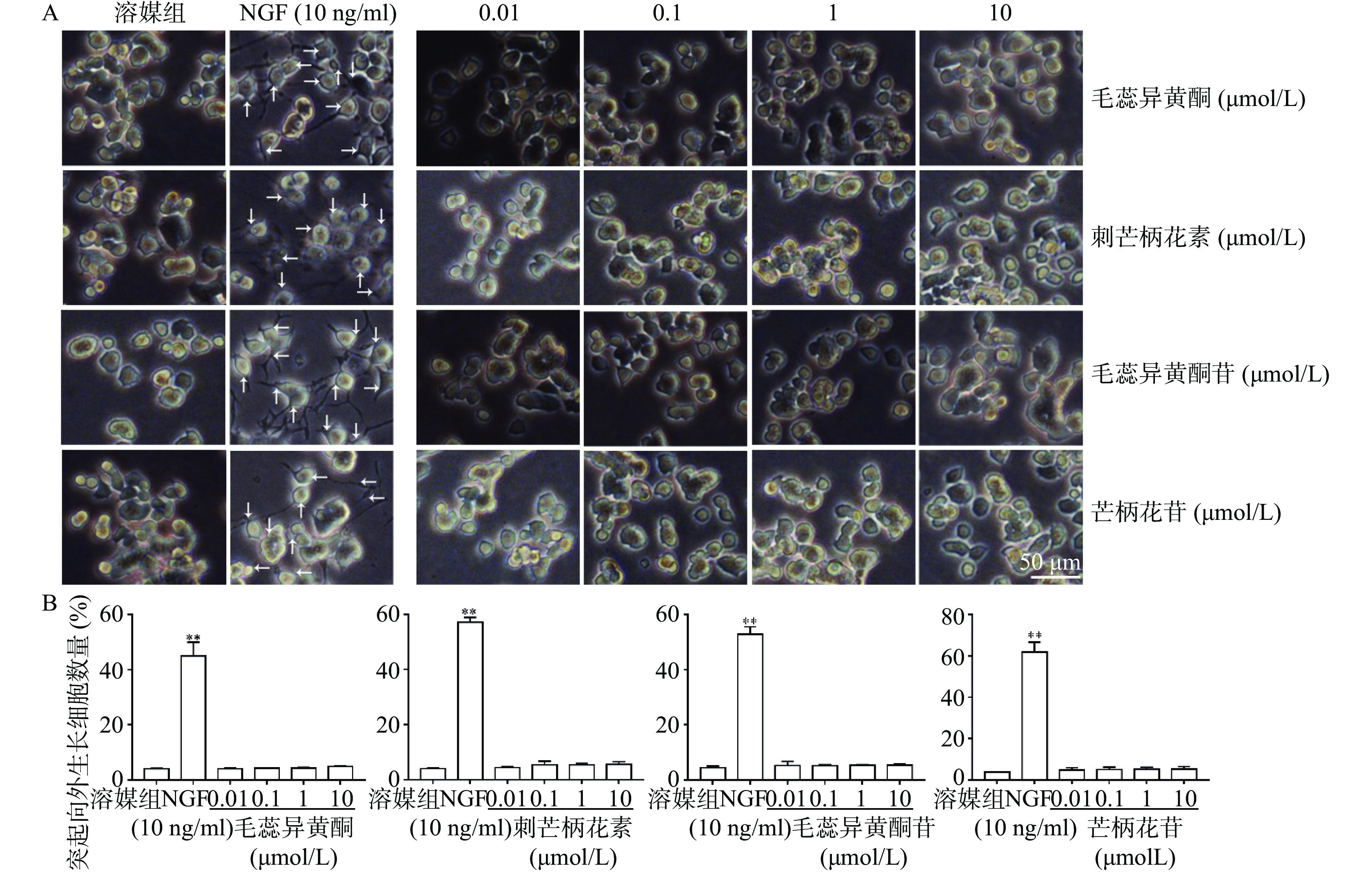
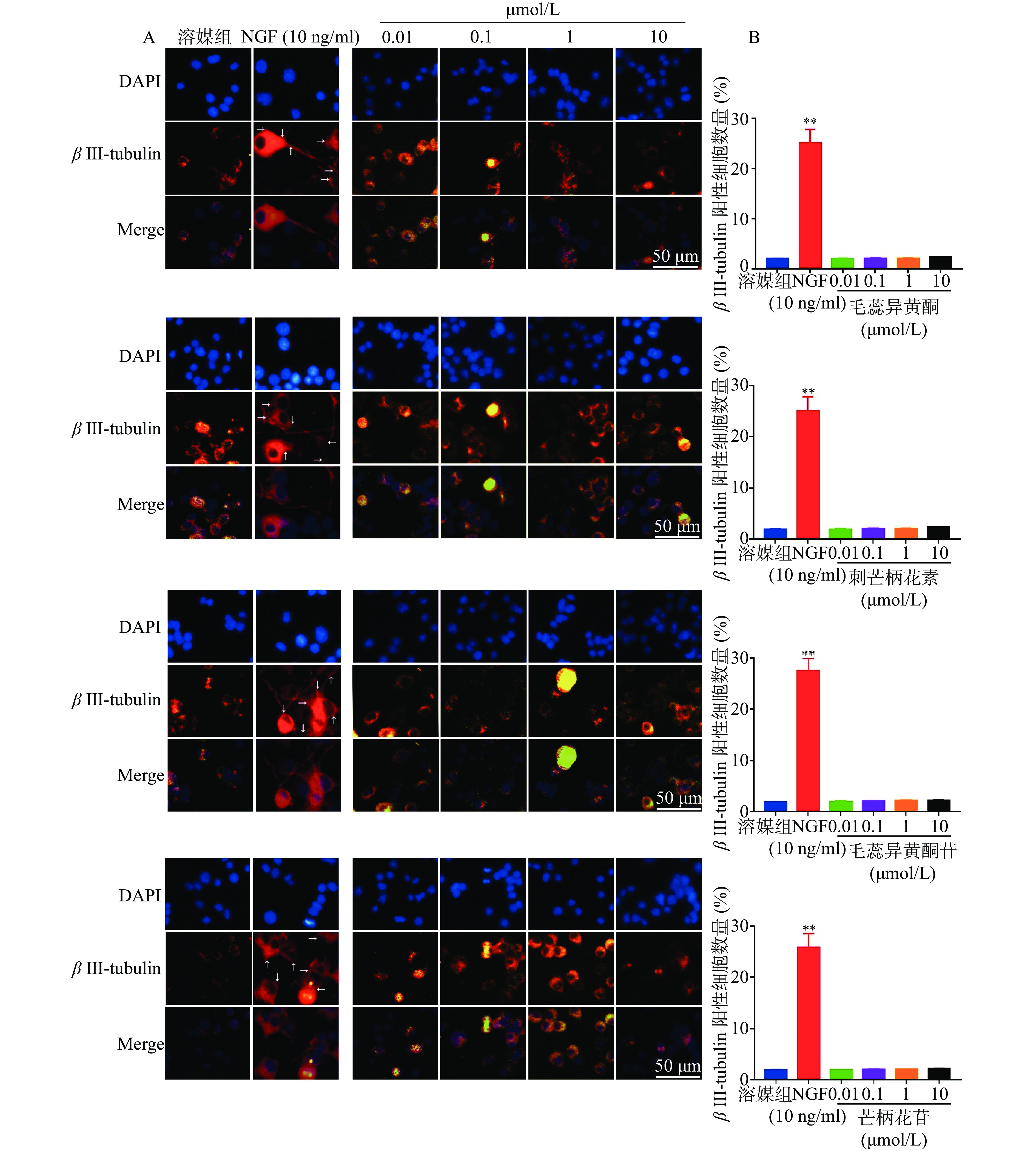
如图3所示,与溶媒组相比,10 ng/ml NGF能显著促进PC 12细胞突起向外生长。与溶媒组相比,毛蕊异黄酮、刺芒柄花素、毛蕊异黄酮苷、芒柄花苷(0.01~10 μmol/L)对PC 12细胞突起向外生长无促进作用。
-
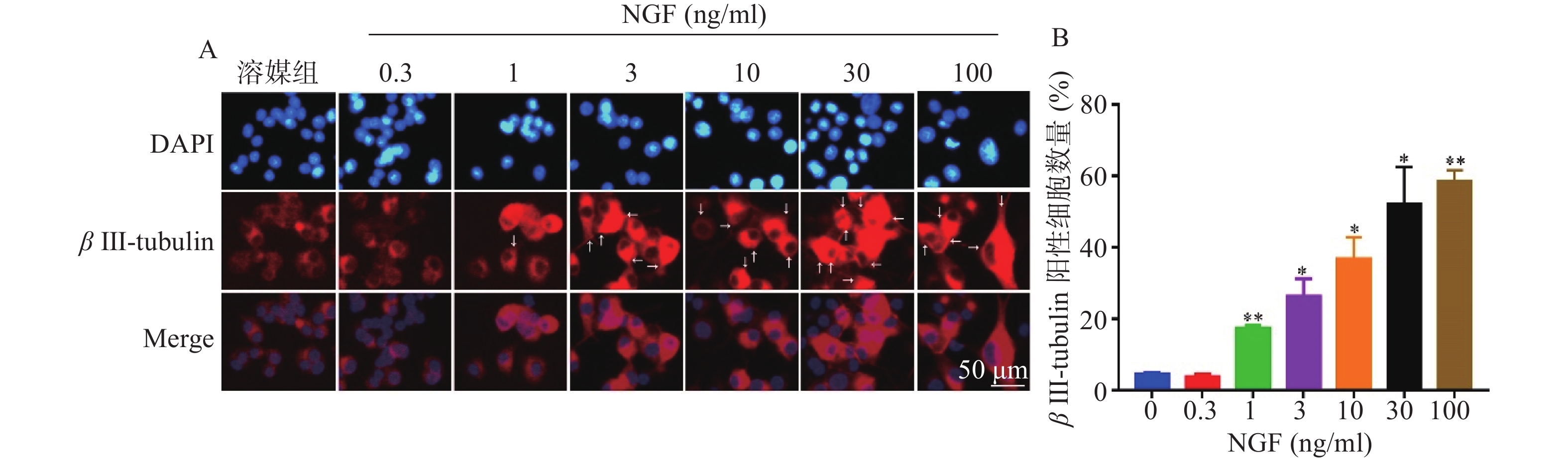
如图4所示,与溶媒组相比,0.3 ng/ml NGF对PC 12细胞中β III-tubulin蛋白表达无影响。与溶媒组相比,NGF(1~100 ng/ml)显著促进PC 12细胞中β III-tubulin蛋白的表达,且具有浓度依赖性。
-
如图5所示,与溶媒组相比,10 ng/ml NGF能显著增加PC 12细胞中β III-tubulin蛋白的表达。与溶媒组相比,毛蕊异黄酮、刺芒柄花素、毛蕊异黄酮苷、芒柄花苷(0.01~10.00 μmol/L)对PC 12细胞中β III-tubulin蛋白表达无影响。
2.1. 不同浓度NGF对PC 12细胞突起向外生长的影响
2.2. 不同浓度及黄芪异黄酮类化合物对PC 12细胞突起向外生长的影响
2.3. 不同浓度NGF对PC 12细胞中β III-tubulin蛋白表达的影响
2.4. 不同浓度黄芪异黄酮类化合物对PC 12细胞β III-tubulin蛋白表达的影响
-
已有文献报道毛蕊异黄酮、刺芒柄花素、毛蕊异黄酮苷和芒柄花苷对急性脑缺血具有神经保护作用,通过抗自噬、抗凋亡、抗炎等发挥作用[7-8, 12-15]。毛蕊异黄酮、刺芒柄花素、毛蕊异黄酮苷和芒柄花苷对神经元分化、缺血后脑损伤神经功能的作用很少提及。本研究初步探讨了毛蕊异黄酮、刺芒柄花素、毛蕊异黄酮苷和芒柄花苷对PC 12细胞神经分化的影响,发现其未能诱导PC 12细胞分化。
在特定因素刺激下,神经元的突起向外生长,促进受损神经元的分化,重新建立神经网络,恢复受损的神经功能[16]。β III-tubulin在微管元件中只表示神经元,是神经细胞分化的表型标志物,增加其表达可改变神经元骨架,促进突起向外生长[17-18]。在本研究中,我们主要以PC 12细胞突起向外生长和β III-tubulin蛋白表达作为主要的分化指标。
在本研究中,用NGF(阳性对照)、毛蕊异黄酮、刺芒柄花素、毛蕊异黄酮苷、芒柄花苷分别刺激PC 12细胞,观察药物对细胞突向外生长的影响。利用免疫荧光染色来观察药物对PC 12细胞β III-tubulin蛋白表达的影响。通过以上两方面的初步研究,我们发现毛蕊异黄酮、刺芒柄花素、毛蕊异黄酮苷、芒柄花苷均没有促进PC 12细胞突起向外生长和β III-tubulin蛋白表达。
综上得出结论:毛蕊异黄酮、刺芒柄花素、毛蕊异黄酮苷、芒柄花苷对PC 12细胞分化无促进作用。









 DownLoad:
DownLoad: