-
维生素K(VK),又称凝血维生素,是一类具有叶绿醌生物活性的脂溶性维生素。除具有凝血功能外,维生素K还能促进骨代谢,防治骨质疏松症,天然VK1和VK2(主要活性体为MK4和MK7)可单独或协同其他抗骨质疏松药物治疗骨质疏松症[1-4],人工合成的VK3也有少量抗骨质疏松研究报道[5-6],然而,不同维生素K的抗骨质疏松作用的比较研究非常有限。有研究发现维生素K具有促进骨形成和抑制骨吸收的双向调节机制[7],药理研究发现VK1、MK4和MK7能显著促进小鼠成骨细胞MC3T3-E1增殖和碱性磷酸酶(ALP)活性[8],同时报道VK1、MK4和MK7也能显著抑制RANKL诱导骨髓单核细胞分化的破骨细胞抗酒石酸酸性磷酸酶(TRAP)基因和组织蛋白酶K(CTSK) mRNA表达[9]。临床报道发现VK2能显著降低绝经后骨质疏松的TRAP和CTSK表达[10]。CTSK是骨吸收过程中的关键溶骨活性酶[11],与粘多糖(glycosaminoglycan, GAG),如硫酸软骨素(CSA),形成一种高分子量的复合物,可将胶原蛋白降解。目前尚未有VK对CTSK骨胶原降解的研究报道。本研究以新生大鼠颅盖骨分离成骨细胞和巨噬细胞集落刺激因子(M-CSF)和核因子κB受体活化因子配体(RANKL)诱导骨髓单核细胞的破骨细胞为模型[12],系统比较VK1、MK4、MK7和VK3对成骨细胞增殖和ALP活性、破骨细胞分化、TRAP活性和CTSK降解胶原活性的影响。通过比较不同VK抗骨质疏松作用,对临床选用合适的VK营养补充剂、指导人群VK膳食补充,以满足我国人群骨健康需求具有重要意义。
-
新生3 d的SD大鼠,雌雄不限,购自上海斯莱克实验动物有限公司[SCXK(沪)2012-0002];II型胶原酶、胰蛋白酶、特级胎牛血清、α-MEM培养基(美国Gibco公司);CellTriter-blue试剂(美国Promega™),核刺激因子受体的配体(receptor for activation of nuclear factor kappa B ligand, RANKL,400-30)、巨噬细胞集落刺激因子(macrophage colony-stimulating factor, M-CSF,400-28)购自PEPROTECH公司,淋巴细胞分离液Ficoll-Paque密度梯度液(GE)购自上海生工生物工程技术服务有限公司;TRACP染色试剂盒、Benzyloxycarbonyl-Phe-Arg-7-amido-4-methylcoumarin (Z-FR-MCA)购自日本WAKO公司;L-3-carboxy-trans-2-3-epoxypropionyl-leucylamido-(4guanidino)-butane(E-64)、硫酸软骨素A (CSA)胃蛋白酶、组织蛋白酶K抑制剂奥当卡替(odanacatib, ODN)、VK1、MK4、MK7和VK3,购自Sigma公司;I型胶原(美国Affymetrix公司);牛血清白蛋白、β-甘油磷酸钠、抗坏血酸、多聚甲醛等试剂均为国产分析纯。
-
实验室自建原代成骨细胞培养方法[12]。取新生3 d的SD大鼠5只,在无菌条件下取其颅顶盖骨,去除骨膜、血管及结缔组织,用D-Hanks液冲洗干净,用0.25%的胰蛋白酶37 ℃消化30 min,再用0.05%胰蛋白酶及3 mg/ml的II型胶原酶37 ℃消化1 h,经100目滤网过滤,1 000 r/min离心10 min,即为新鲜的成骨细胞,加入10%胎牛血清的α-MEM培养基,置37 ℃,5% CO2培养箱。取第四代大鼠颅盖骨成骨细胞,消化分离出来,以α-MEM培养基配制成浓度为2×104/ml的细胞悬液,以100 μl/孔接种于96孔培养板,设12个药物组,分别为1、0.1和0.01 μmol/L的VK1、MK4、MK7和VK3,每组选取1个药物浓度加入1、0.1和0.01 μmol/L的VK1、MK4、MK7和VK3,培养48 h,用MTT法检测细胞增殖活性。ALP活性需要连续培养6 d后,用100 μl 50 mmol/L的二乙醇胺,50 μl 2.5 mmol/L的对硝基苯酚磷酸二钠,37 ℃反应30 min后,用0.3 mol/L的NaOH终止反应,于波长405 nm处测得吸光度值[12-13]。
-
取成骨细胞第三代细胞,按每孔5×104的细胞数接种于12孔板内,α-MEM培养基培养24 h,换骨结节诱导培养基(0.1%BSA,10 nmol/L地塞米松,10 mmol/L β-甘油磷酸钠和50 μg/ml抗坏血酸以及10%胎牛血清的α-MEM培养基),加入1 μmol/L的VK1、MK4、MK7和VK3后,每3 d换药1次,连续培养观察14 d后,用0.1%茜素红-Tris-Hcl染液(pH 8.3)染色。染色前,用预冷的10%中性甲醛缓冲液固定10 min,PBS冲洗3次。加入0.1%茜素红-Tris-Hcl染液(pH 8.3),37 ℃下染色30 min。蒸馏水冲洗,干燥,封片。倒置相差显微镜(Leica DMI 3000)下进行观察并随机拍照10张,用image-Pro Plus (IPP 6.0)分析图片,得出每张图片骨结节面积[13]。
-
取新生3 d的SD大鼠胫骨,用α-MEM培养基冲洗骨髓腔以收集骨髓细胞,加入等量Ficoll试剂分离骨髓单核细胞,用含有25 ng/ml M-CSF、25 ng/ml RANKL的细胞因子和10%胎牛血清的α-MEM培养基进行培养,每3 d换液1次,6 d后破骨细胞分化成熟。细胞成熟后,以1×105/ml接种于96孔板,加入1、0.1和0.01 μmol/L的VK1、MK4、MK7和VK3。继续培养48 h后,取100 μl培养基,加入20 μl CellTriter-blue试剂,轻轻晃10 s混匀,37 ℃孵育30 min后,将96孔板置于荧光分光光度计,检测细胞悬液荧光强度(发散光波长560 nm,吸收光波长590 nm)。
-
成熟破骨细胞以1×105/ml接种于96孔板内,加入1、0.1和0.01 μmol/L的VK1、MK4、MK7和VK3,培养48 h后,弃上清液,PBS冲洗2次,20 µl 0.1%Triton X-100室温破碎细胞15 min,加入100 µl反应液(0.4 g对硝基苯基磷酸二钠,去离子水溶解后加入2.0 g酒石酸钾钠,加水溶解至150 ml,HCl调节pH至3.5,再加水定容至200 ml),于37 ℃反应30 min,迅速加入100 µl 1 mol/L的NaOH终止反应,于波长405 nm处测定其吸光度值[14]。
-
25 μmol/L的VK1、MK4、MK7、VK3和1 μmol/L阳性药Odanacatib用100 mmol/L醋酸钠缓冲液(pH5.5,包含2.5 mmol/L DTT,和2.5 mmol/L EDTA)稀释至浓度为25 μmol/L加入96孔板,最终反应容量为0.2 ml,加入终浓度为5 nmol/L的CTSK孵育5 min,加入5 μl底物1 mmol/L的Z-FR-MCA溶液开始反应,检测5 min荧光信号(发散光波长460 nm,吸收光波长355 nm)。实验设阴性对照,阳性对照(1 μmol/L E-64)。
计算公式:抑制率(%)=100-(1-Vi/V0)。
Vi和V0分别表示存在和不存在VK情况下,记录荧光信号强度随时间变化斜率slope值[11]。
-
可溶性I型胶原溶解在100 mmol/L醋酸钠缓冲液(pH5.5,包含2.5 mmol/L DTT和2.5 mmol/L EDTA),最终I型胶原浓度为0.6 mg/ml,加入终浓度100 μmol/L的VK1、MK4、MK7和VK3及10 μmol/L阳性对照药Odanacatib,依次将CTSK和CSA(终浓度分别为400和200 nmol/L)加入含有I型胶原蛋白的反应液中,使总反应液容量为50 μl。混匀,28 ℃孵育4 h,取出后加入1 μl的100 μmol/L E64终止反应。采用10%的SDS-PAGE凝胶电泳分离,卡马斯亮蓝染色20 min,用乙酸-甲醇(4∶1)脱色。I型胶原的α1条带的灰度用Gene Snap(Syngene Inc.Frederick,MD)软件进行定量分析[14]。
-
每组实验重复3次。采用SPSS软件经ANOVA方差分析检验(α=0.05),再用SNK对每两组进行两两比较,以P<0.05为差异有统计学意义。
-
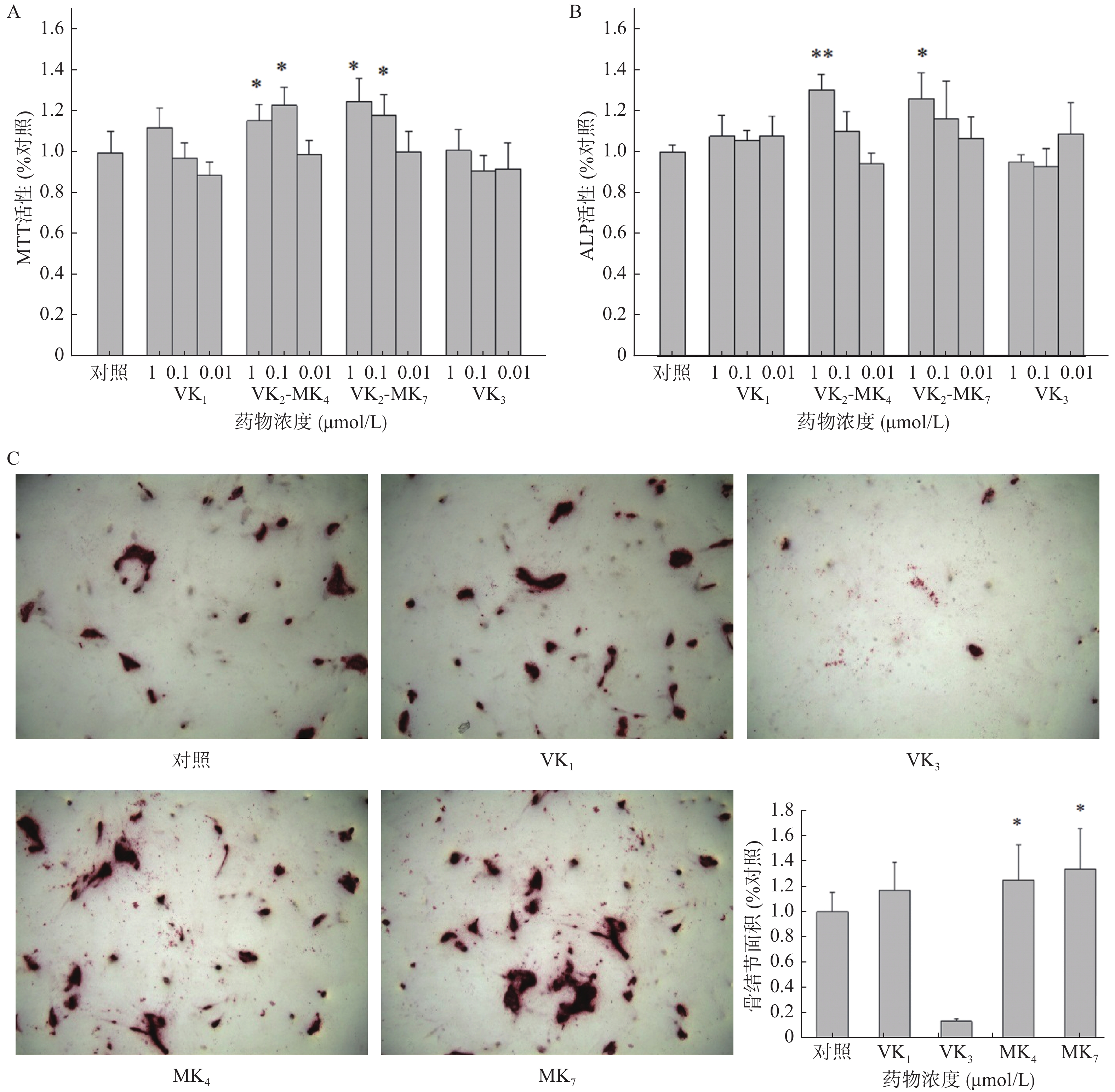
VK1和VK3在0.01~1 μmol/L浓度范围内对成骨细胞增殖和ALP活性均未有影响(图1A、1B)。MK4在0.01和0.1 μmol/L浓度显著促进了成骨细胞增殖活性,分别提高了15.5%和23.0%(P<0.05),而MK7分别提高了25.1%和18.4%。MK4和MK7在1 μmol/L显著提高了ALP活性(P<0.05),促进率分别为30.2%和25.7%。成骨细胞在骨结节诱导培养基培养14 d后,经茜素红染色液染色,骨结节处形成红色钙化晶体,骨结节的面积代表了成骨细胞的钙化程度(图1C),结果显示MK4和MK7在1 μmol/L浓度时显著提高了骨结节形成面积(P<0.05),分别增加25.2%和34.0%,VK3显著抑制了骨结节的形成。
-
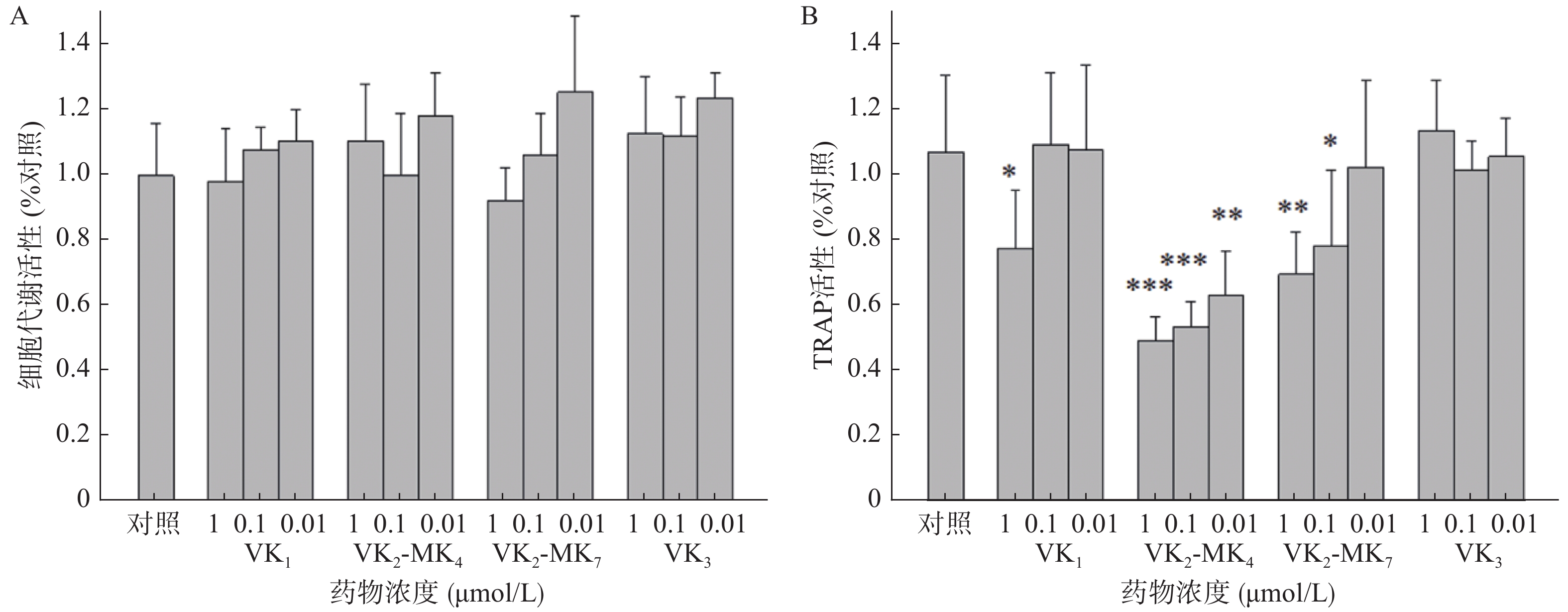
由图2A可得,VK1、VK3、MK4和MK7在0.01~1 μmol/L浓度对破骨细胞代谢活力均无影响,对破骨细胞无毒性。VK1、MK4和MK7在1 μmol/L表现出抑制TRAP活性,抑制率分别为27.4%、54.0%和35.0%,MK4在0.01和0.11 μmol/L浓度显著降低了破骨细胞TRAP活性(P<0.01),分别降低了50%和41%,MK7在0.1 μmol/L浓度时,TRAP活性显著降低26.8%(图2B)。
-
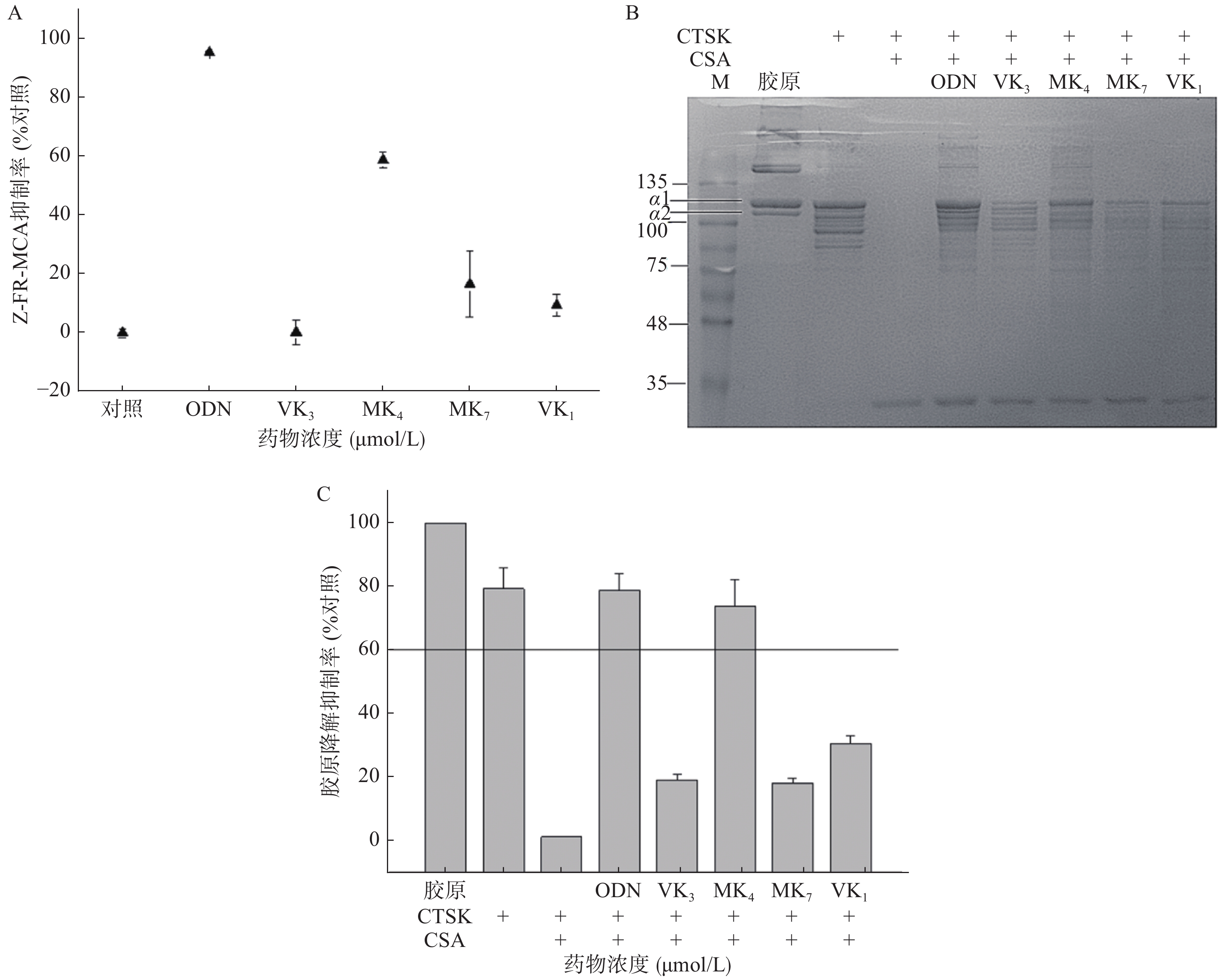
本研究考察VK1、VK3、MK4和MK7抑制CTSK与Z-FR-MCA底物结合活性。图3A结果显示,1 μmol/L的ODN抑制了CTSK与Z-FR-MCA底物结合效率达98.3%,而25 μmol/L的VK3、MK4、MK7和VK1抑制率分别为0.3%、58.9%、16.6%和9.4%。与空白胶原相比,VK3、MK4、MK7和VK1在100 μmol/L抑制胶原降解效率达30.8%、73.8%、18.4%和19.2% (图3B、3C),仅有MK4和ODN超过60%的抑制率,与未加CSA的CTSK阴性对比,抑制率达38.8%、93.0%、23.1%和24.2%。与空白胶原组对比,阳性抑制剂ODN在1 μmol/L的抑制率达77.3%,与未加CSA的CTSK阴性对比,抑制率达到99.0%。
-
研究报道VK1、MK4和MK7能显著促进成骨细胞活性和抑制破骨细胞活性[9-10]。临床报道服用高含量维生素K1能够降低髋部骨折风险和提高髋部及腰椎部骨密度,可延缓50~60岁绝经后女性的骨丢失[2]。同时药理研究发现VK3显著促进去卵巢大鼠的骨密度和改善骨质疏松相关指标[5]。而本研究并未发现VK1和VK3具有促进成骨细胞和抑制破骨细胞的作用,可能原因是选择的筛选浓度过低所致。MK4和MK7在1 μmol/L能促进成骨细胞增殖和矿化作用,二者并没有显著差异。但在抑制骨吸收作用方面,MK4相较于MK7在相同浓度,有更显著的抑制TRAP活性的作用。
CTSK是破骨细胞发挥骨吸收作用的关键靶标酶,选择性地大量表达于破骨细胞,其生理作用底物正是在有机骨基质中含量达95%的I型胶原[11]。我们研究发现,在相同浓度,只有MK4能显著抑制其底物骨胶原降解和人工合成底物Z-FR-MCA的活性。
日本早在1995年首次将MK4作为治疗骨质疏松的药物应用[15],2011年MK4录入我国《原发性骨质疏松症诊疗指南》[16]。因此,我们认为MK4是人体内源性营养物质,安全性高,适用于各年龄人群作为营养补充剂使用。MK7具有促进骨形成作用,在体内也可以分解成MK4发挥作用,适合作为营养食品补充剂。
Effects of vitamin K on osteoblastic bone formation and osteoclastic bone absorption
doi: 10.12206/j.issn.1006-0111.202001077
- Received Date: 2020-01-17
- Rev Recd Date: 2020-04-13
- Available Online: 2020-07-27
- Publish Date: 2020-07-25
-
Key words:
- vitamin K /
- osteoporosis /
- osteoblasts /
- osteoclasts
Abstract:
| Citation: | JIANG Yizhong, XIA Tianshuang, XIN Hailiang, JIN Yu'e, JIANG Yiping, XUE Liming. Effects of vitamin K on osteoblastic bone formation and osteoclastic bone absorption[J]. Journal of Pharmaceutical Practice and Service, 2020, 38(4): 340-345. doi: 10.12206/j.issn.1006-0111.202001077 |








 DownLoad:
DownLoad: