-
中药代煎使用自动煎药机,机械化生产效率高,加水量、煎药时间容易控制,能做到煎药规范化、标准化、数字化管理[1-2]。中药处方中经常有“先煎”、“后下”的药材,需要特殊煎法时特别处理,一般先按要求煎好,分装后保存,用时加入到复方中混合。提取液的放置环境温度及时间长短都会影响到有效成分的稳定性从而降低复方汤剂的药效。目前中药“后下”品种预煎液尚无完整的质量控制过程,缺少有效的质量监管措施,难以保证中药代煎液临床疗效的一致性。
肉桂为樟科(Lauraceae)樟属植物肉桂(Cinnamomum cassia Presl)的干燥树皮,因含有丰富的芳香族、萜类、脂肪族等挥发油化合物,长时间煎煮会导致药性损失,故中药传统煎煮方法将其作为“后下”品种。肉桂酸作为肉桂主要的药效成分,具有抗菌、抗癌、发汗等作用[3-5]。本研究以“后下”品种肉桂预煎液为例,以有效成分含量变化作为观察指标,采用HPLC法测定有效成分肉桂酸的含量,研究不同储存温度及时间对肉桂袋装预煮液质量稳定性的影响,并为今后制订“后下”中药预煎液储存规范提供一定的科学依据。肉桂酸有多种含量测定方法,包括HPLC法[6]、近红外定量校正法[7]、液相色谱串联质谱法[8]等,其中,HPLC法具有较多优点,如速度快、分辨率高等[9]。因此,笔者首先考虑使用HPLC法测定肉桂预煎液中有效成分肉桂酸的含量。
HTML
-
美国Agilent公司1100系列HPLC仪(在线脱气机、四元泵、自动进样器、柱温箱、二极管阵列检测器);瑞士梅特勒-托利多公司METYLER AE240型十万分之一电子天平;上海科导超声仪器公司SB 3200-T超声发生器。
-
肉桂酸对照品(批号:6648,上海诗丹德生物科技有限公司);甲醇和乙腈均为色谱纯(美国Merck 公司),甲酸为色谱纯(SIGMA 公司),水为超纯水;肉桂预煎液(批号:191213,上海同济堂药业)。
1.1. 仪器
1.2. 试药
-
色谱柱为Agilent Zorbax C18柱(4.6 mm×250 mm,5 μm),流动相:0.1%甲酸溶液-乙腈(60:40),比例流速1.0 ml/min,柱温25 ℃;进样量5 μl;检测波长:275 nm,分析时间:16 min。
-
精密称取肉桂酸对照品10.21 mg,置于10 ml容量瓶中,加甲醇溶解并稀释至刻度,摇匀,得浓度为1.021 mg/ml的肉桂酸对照品储备液,置冰箱4 ℃保存。
-
取适量肉桂预煎液,12 000× g离心5 min,取上清液滤过,取续滤液,即得。
-
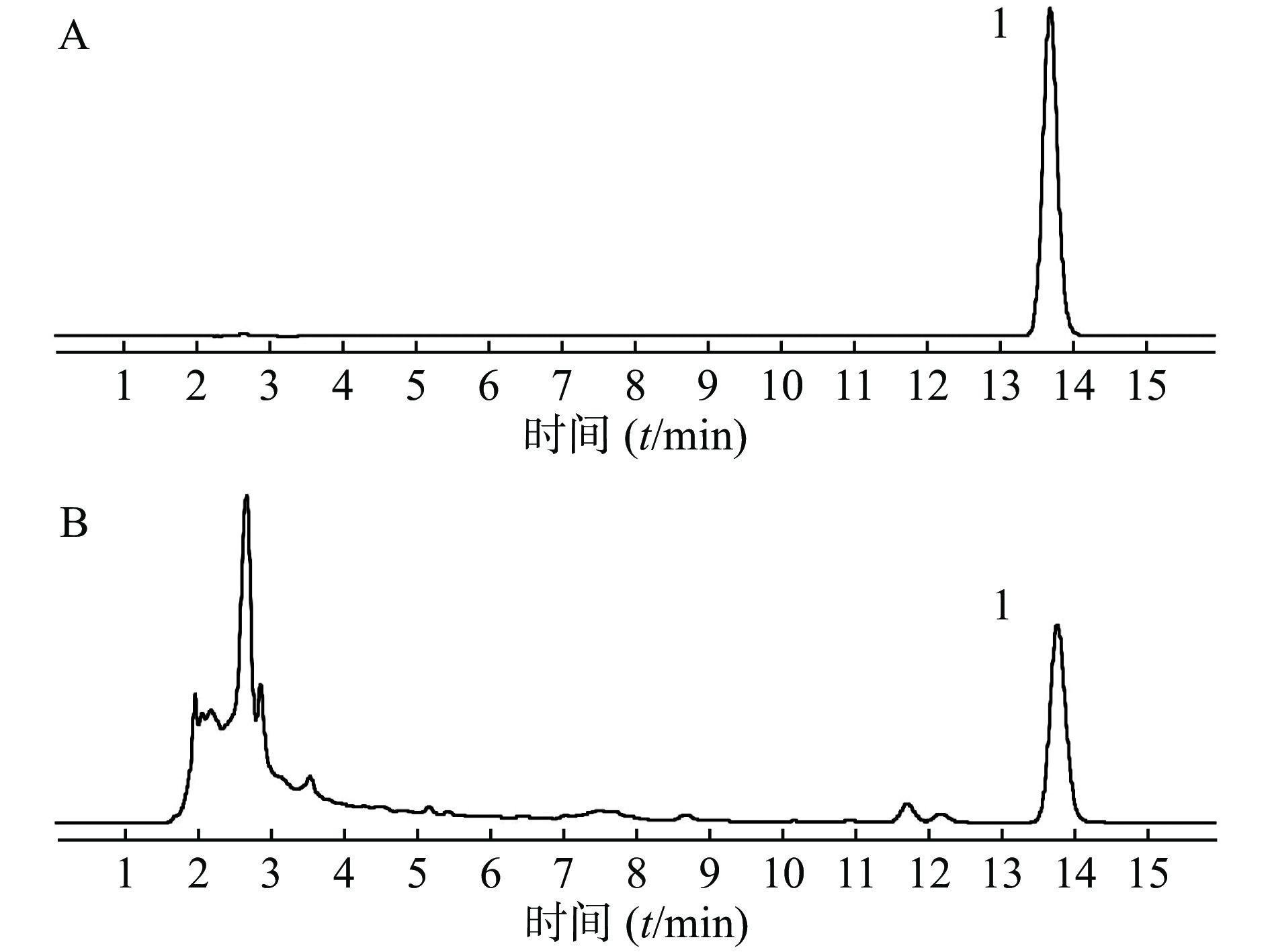
取供试品和对照品溶液,按“2.1 色谱条件”项下进样分析,得液相色谱图,见图1,肉桂酸的保留时间为13.8 min,色谱峰分离度均大于1.5,理论塔板数大于5 000。结果表明,肉桂酸色谱峰与其他成分色谱峰得到较好的分离。
-
采用逐级稀释法,精密量取适量对照品溶液,置于10 ml容量瓶中,加入甲醇定容,得到肉桂酸的浓度分别为10.21、20.42、51.05、102.10、204.20 μg/ml系列的对照品溶液,按“2.1”项下进样。以浓度为横坐标(X),色谱峰面积值为纵坐标(Y)进行线性回归,得到线性回归方程:Y=48.32X+8.819,r=0.999 9。结果表明肉桂酸在10.21~204.20 μg/ml范围内线性良好。
-
取低、中、高3个浓度(20.42、51.05、102.10 μg/ml)对照品溶液,按“2.1”项下进样分析,1 d内重复测定3次,连续3 d测定,记录色谱峰面积,考察日内和日间精密度,结果肉桂酸日内精密度RSD值分别为0.36%、0.96%、0.81%(n=3),日间精密度RSD值分别为1.67%、1.20%、1.10%;结果显示该方法精密度符合方法学要求,仪器精密度良好。
-
取同一批次肉桂预煎液,共6份,按“2.2.2”项下方法操作制备供试品溶液,按“2.1”项下进样分析,记录峰面积,结果肉桂酸预煎液中肉桂酸峰面积的RSD为1.12%,表明该方法的重复性良好。
-
按“2.2.2”项下方法操作制备供试品溶液,分别在0、4、8、12、24、48 h测定供试品溶液中肉桂酸的含量,考察供试品常温下放置稳定性,肉桂酸RSD为1.13%,结果表明样品溶液在48 h内稳定性良好。
-
精密吸取1 ml肉桂预煎液,置于含51.05 μg肉桂酸(氮气挥干)离心管中,涡旋30 s,平行操作5份,按“2.2.2”项下方法操作制备供试品溶液,按“2.1”项下进样分析,计算加样回收率,结果如表1所示,肉桂酸的平均加样回收率为98.9%(RSD=1.9%,n=5),结果表明该方法回收率良好。
样品含量(m/μg) 对照液加入量(m/μg) 测得量(m/μg) 回收率(%) 平均回收率(%) RSD(%) 47.68 51.05 97.02 96.7 98.9 1.9 47.68 51.05 97.64 97.9 47.68 51.05 97.89 98.4 47.68 51.05 99.09 100.7 47.68 51.05 99.30 101.1 -
取3袋肉桂预煎液于当日(第0天)测定肉桂酸的含量,按“2.2.2”项下方法操作制备溶液,按“2.1”项下进样分析,以峰面积外标法计算含量。剩余袋装药液分组保存在4 ℃(冷藏组)、25 ℃(常温组)、40 ℃(高温组)恒温条件下,1、3、7、14、21、30 d后重复上述步骤,检测肉桂酸的含量。应用SPSS 19.0软件进行统计分析,数据以(
${\overline x} \pm{s} $ )表示。多组间差异比较采用单因素方差分析,两组间差异比较采用LSD法,检验水准(α)为 0.05。结果如表2所示,与储存第0天相比,常温储存21、30 d,高温温储存14、21、30 d测得的肉桂酸含量均显著降低(P<0.01),其他组未发现明显变化(P>0.05)。组别 储存时间(t/d) 0 1 3 7 14 21 30 冷藏组(4 ℃) 47.27±0.66 47.09±0.73 47.64±1.08 46.51±0.65 46.13±0.73 46.33±0.68 46.28±1.14 常温组(25 ℃) 47.27±0.66 47.66±0.27 47.54±1.14 47.62±0.63 47.15±0.69 42.49±0.54** 38.02±0.60** 高温组(40 ℃) 47.27±0.66 47.30±0.22 47.18±0.61 47.07±0.69 44.38±0.66** 38.90±0.41** 33.31±0.56** ** P<0.01,与第0天比较。
2.1. 色谱条件
2.2. 溶液的制备
2.2.1. 对照品溶液
2.2.2. 供试品溶液
2.3. 系统适用性试验
2.4. 线性关系考察
2.5. 精密度试验
2.6. 重复性试验
2.7. 稳定性试验
2.8. 加样回收率试验
2.9. 肉桂酸长期稳定性试验
-
中药材从固体饮片变为液体煎剂,质量处于不稳定状态,其后期的质控和管理显得尤为重要[10]。本实验以本院常用“后下”品种肉桂为例,建立有效成分肉桂酸的HPLC含量测定方法,在所确定的实验条件下,该分析方法快速、准确、方便;应用该方法对其不同放置条件和时间的稳定性进行系统考察,为制订本院“后下”品种预煎液内部质量控制标准提供了实验依据。
预实验中比较了水、甲醇、乙腈、甲酸、铵盐溶液等不同的流动相体系,最终选择0.1%甲酸-乙腈(60:40)作为流动相进行等度洗脱,样品中肉桂酸的待测成分峰形较好,与其他成分可达到基线分离。通过DAD进行全波长扫描分析肉桂酸的紫外光谱,275 nm波长下肉桂酸有较高吸收峰,出峰处无干扰成分,因此选用275 nm作为检测波长。
将有效成分含量变化作为观察指标,兼顾普通患者家庭环境温度,分析考察放置环境和时间对肉桂预煎液稳定性的影响。结果发现冷藏(4 ℃)储存的药液在30 d内基本稳定;常温(25 ℃)储存21、30 d后,肉桂酸的浓度显著下降,分别降到最初浓度的89.9%和80.4%;高温(40 ℃)储存14、21、30 d后,肉桂酸的浓度分别降到最初浓度的93.7%、82.3%和70.5%。由此可见,低温保存肉桂酸较稳定,冷藏有利于肉桂预煎液的稳定性。
综上,本实验建立的分析方法为肉桂预煎液的质量控制提供了一种可靠的方法,为后续制订含量的质量控制范围奠定了基础,并为今后制订“后下”中药预煎液储存规范提供了一定的科学依据。









 DownLoad:
DownLoad: