-
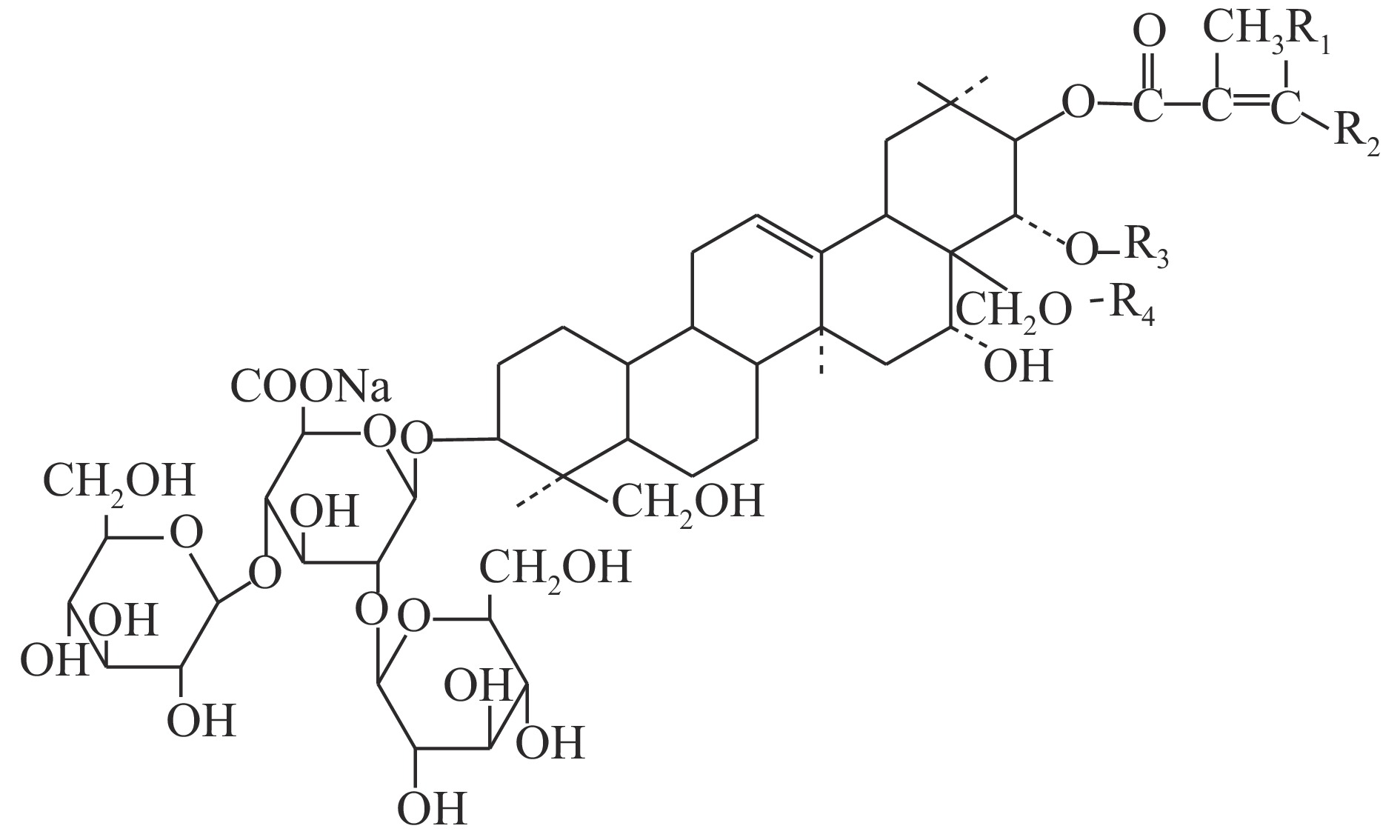
七叶皂苷钠(sodium aescinate)是一种含酯键三萜皂苷的钠盐,呈白色或类白色结晶粉末,味苦而辛,提取自七叶树植物天师栗(Aesculus wilsonii Rehd.)的干燥成熟种子,主要有效成分为七叶皂苷A、B、C和D[1](见图1和表1),具有消炎、抗渗出、增加静脉张力、改善血液循环以及纠正脑功能失常等作用[2]。七叶皂苷钠在药物类别上按化学类药品管理,制剂主要包括注射用七叶皂苷钠、七叶皂苷钠片、复方七叶皂苷钠凝胶和七叶皂苷钠搽剂。注射用七叶皂苷钠及原料药的质量标准收载于《国家药品西药标准(化学药品地标升国标第十六册)》[3];复方七叶皂苷钠凝胶和七叶皂苷钠搽剂的质量标准为新药转正标准;七叶皂苷钠片质量标准执行国家食品药品监督管理局标准。
名 称 R1 R2 R3 R4 七叶皂苷钠A(β) —CH3 —H —COCH3 —H 七叶皂苷钠B(β) —H —CH3 —COCH3 —H 七叶皂苷钠C(α) —CH3 —H —H —COCH3 七叶皂苷钠D(α) —H —CH3 —H —COCH3 目前,七叶皂苷钠的测定方法主要有HPLC法、紫外分光光度法、薄层色谱法和酸碱滴定法[4]。其中,国家药品标准中规定的含量测定方法为酸碱滴定法,该法需要4 h以上的加热回流时间,且前处理过程复杂,而操作较简便的紫外分光光度法及薄层色谱法灵敏性和选择性又相对较差。因此,建立一种操作简便、选择性好的七叶皂苷钠快检方法,对于该药物的质量安全监管具有重要意义。近红外光谱分析法(near infrared spectrum,NIRS)是一种间接分析技术,通过建立目标物属性值与近红外光谱数据之间的关联模型,对待测样品进行定性或定量检测[5-6]。所建模型的质量常用决定系数(R2)和交叉验证均方根误差(RMSECV)等参数进行评价,R2值越接近1,说明样品测定值与预测值相关性越好;RMSECV则决定了预测样品的误差大小,应与实验室标准方法的精密度相当。与传统分析技术相比,具有分析速度快、成本低、效率高、重现性好等优点,可用于药品的在线无损分析[7-8]。
本文利用近红外光谱分析技术,建立了一种七叶皂苷钠的快检方法,用于注射用七叶皂苷钠药品的含量测定。本方法根据OPUS软件对采集光谱进行了优化处理,分别采用PLS算法和因子化算法,建立七叶皂苷钠的定量模型和定性模型。
HTML
-
试验使用A药厂43批次注射用七叶皂苷钠样品;B药厂4批次注射用盐酸乌拉地尔样品;C药厂4批次注射用胸腺五肽样品;D药厂4批次注射用七叶皂苷钠样品;试验用试剂均为分析纯,购自国药集团。
-
试验采用德国Bruker公司的MPA近红外光谱仪,装载OPUS软件;上海喆图公司的电热恒温水浴锅。
1.1. 试药
1.2. 仪器
-
取本品30支(10 mg取15支),加乙醇-水(10∶1)溶解,定量转移至25 ml量瓶中,并稀释至刻度,摇匀,精密量取10 ml至100 ml三角瓶中。加酚酞指示液2滴,用氢氧化钠溶液(0.01 mol/L)滴定至溶液显微红色后,精密加氢氧化钠滴定液(0.05 mol/L)5 ml,加热回流4 h,用乙醇-水(10∶1)2 ml洗涤冷凝管及瓶口,取出,放冷至室温,用盐酸滴定液(0.01 mol/L)滴定,并将滴定的结果用空白实验校正。每1 ml的氢氧化钠滴定液(0.05 mol/L)相当于28.80 mg的七叶皂苷钠。
-
近红外光谱检测采用光纤扫描方法,分辨率为8 cm−1,样品扫描时间为16 s,背景扫描时间为32 s,扫描范围为12500 cm−1~4000 cm−1。将光纤探头对准被测样品西林瓶底部(样品应均匀分布在西林瓶底部,保证探头扫面处样品均匀),测定3次,计算平均光谱。
-
B药厂4批次注射用盐酸乌拉地尔的4张光谱;C药厂4批次注射用胸腺五肽的4张光谱;D药厂4批次注射用七叶皂苷钠的4张光谱。
-
采集上述样品的近红外光谱,经一阶导数和矢量归一化法处理,平滑点数为9,采用因子化法,设定谱段为9037.3 cm−1。
-
用胸腺五肽定性模型,鉴定B药厂注射用盐酸乌拉地尔、C药厂注射用胸腺五肽和D药厂注射用七叶皂苷钠样品,结果如表2。结果表明,该定性模型可准确区分未参与建模的3个品种样品,可用于A药厂生产的注射用七叶皂苷钠定性检测。
样品名称 鉴定结果 鉴定结论 匹配数 匹配值 阈值 组名称 注射用乌拉地尔 1 0.01265 0.02330 注射用乌拉地尔 样品为注射用乌拉地尔 2 0.68066 0.01932 注射用七叶皂苷钠 3 1.23027 0.01235 注射用胸腺五肽 注射用胸腺五肽 1 0.00701 0.01235 注射用胸腺五肽 样品为注射用胸腺五肽 2 0.55662 0.01932 注射用七叶皂苷钠 3 1.24993 0.02330 注射用乌拉地尔 注射用七叶皂苷钠 1 0.00879 0.01932 注射用七叶皂苷钠 样品为注射用七叶皂苷钠 2 0.55840 0.01235 注射用胸腺五肽 3 0.68452 0.02330 注射用乌拉地尔 -
共采用A药厂的43批次注射用七叶皂苷钠样品。其中,38批次样品用于建立定量模型,5批样品用于模型验证。
-
采集样品的近红外光谱,求出平均光谱,根据OPUS7.8软件提供的优化结果,选择一阶导数和矢量归一化,以消除样品的基线偏移,降低背景及噪声干扰,提高信噪比,增强光谱特征。采用PLS算法,利用软件的预处理选择合适的谱段建模,得到注射用七叶皂苷钠含量测定校正模型,最后采用内部交叉算法,对模型进行验证。
-
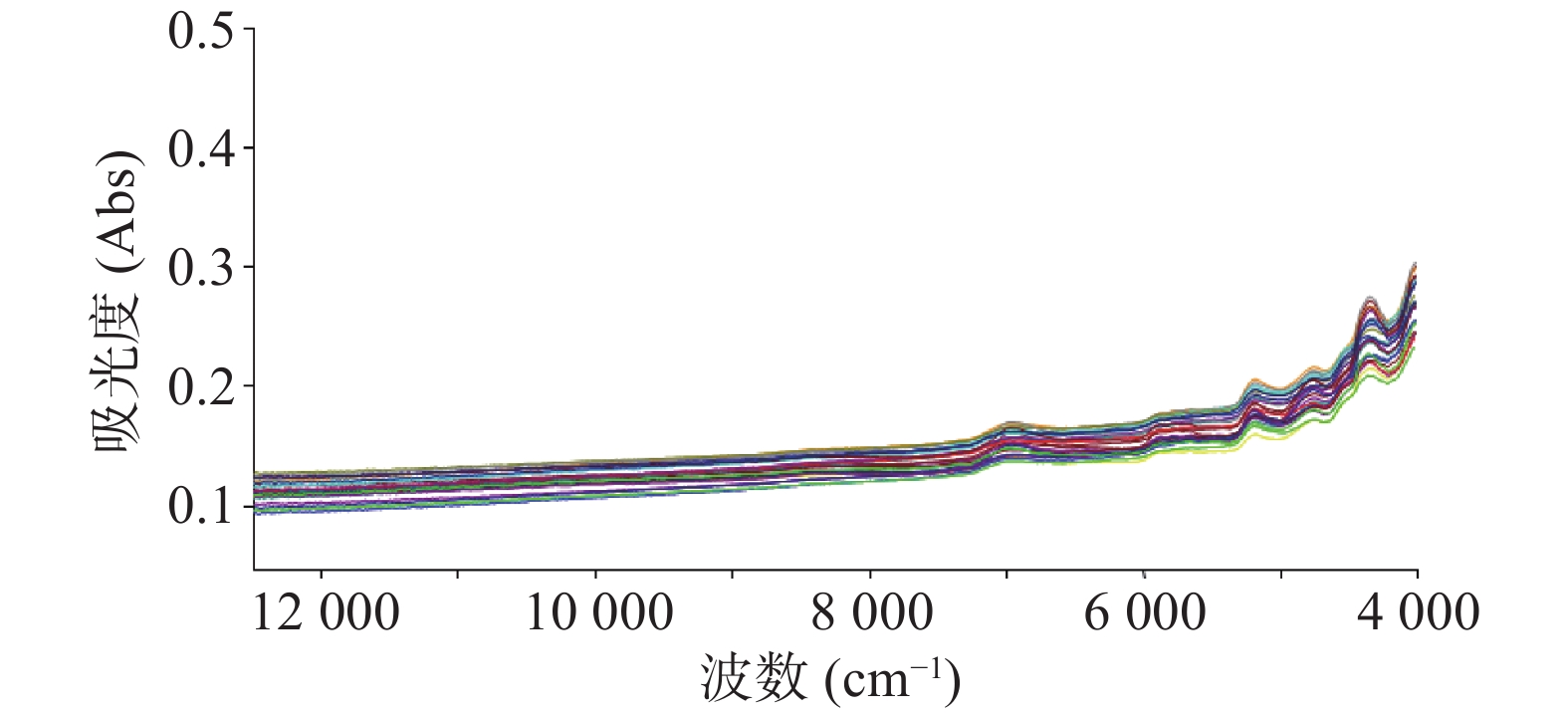
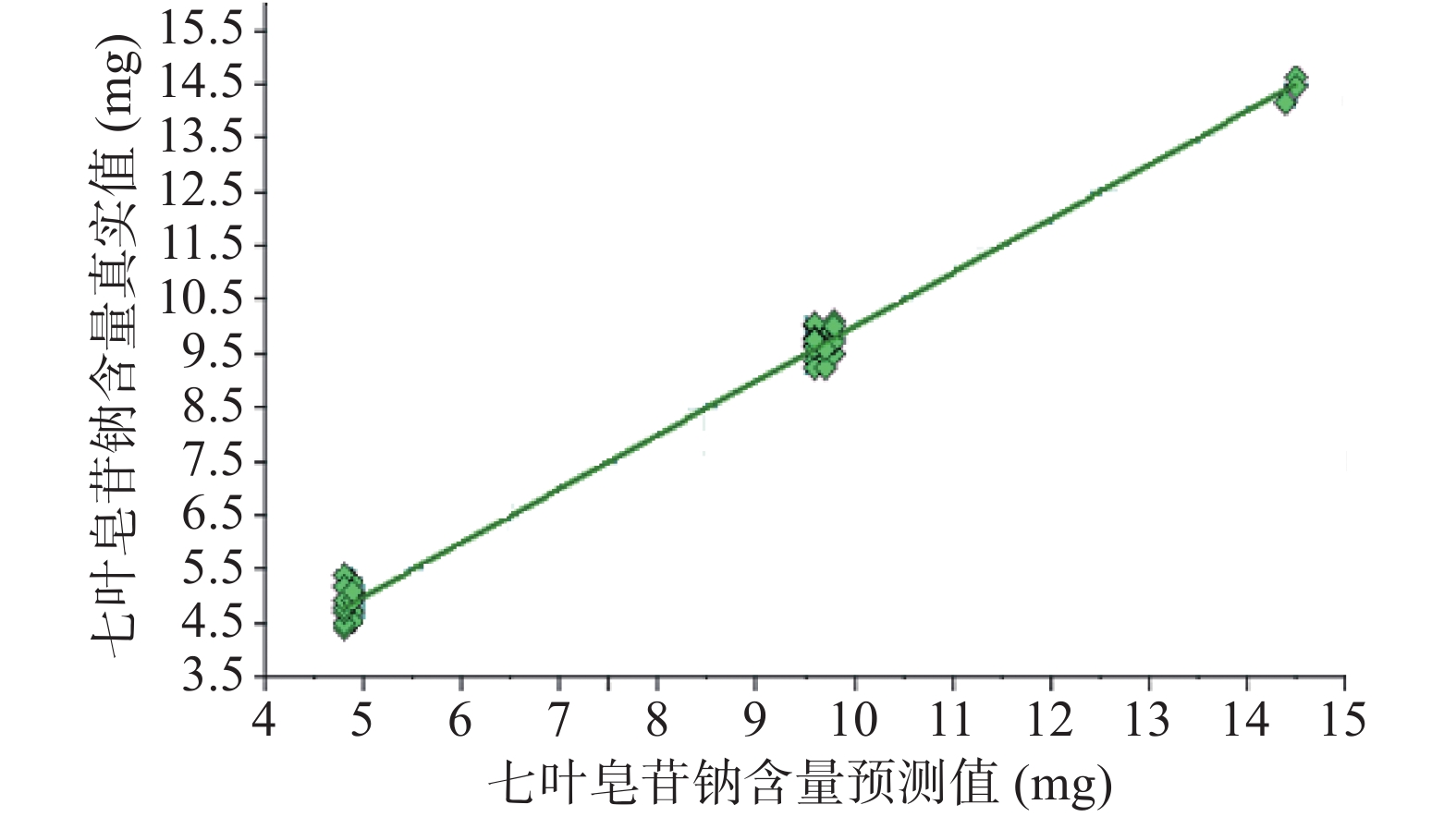
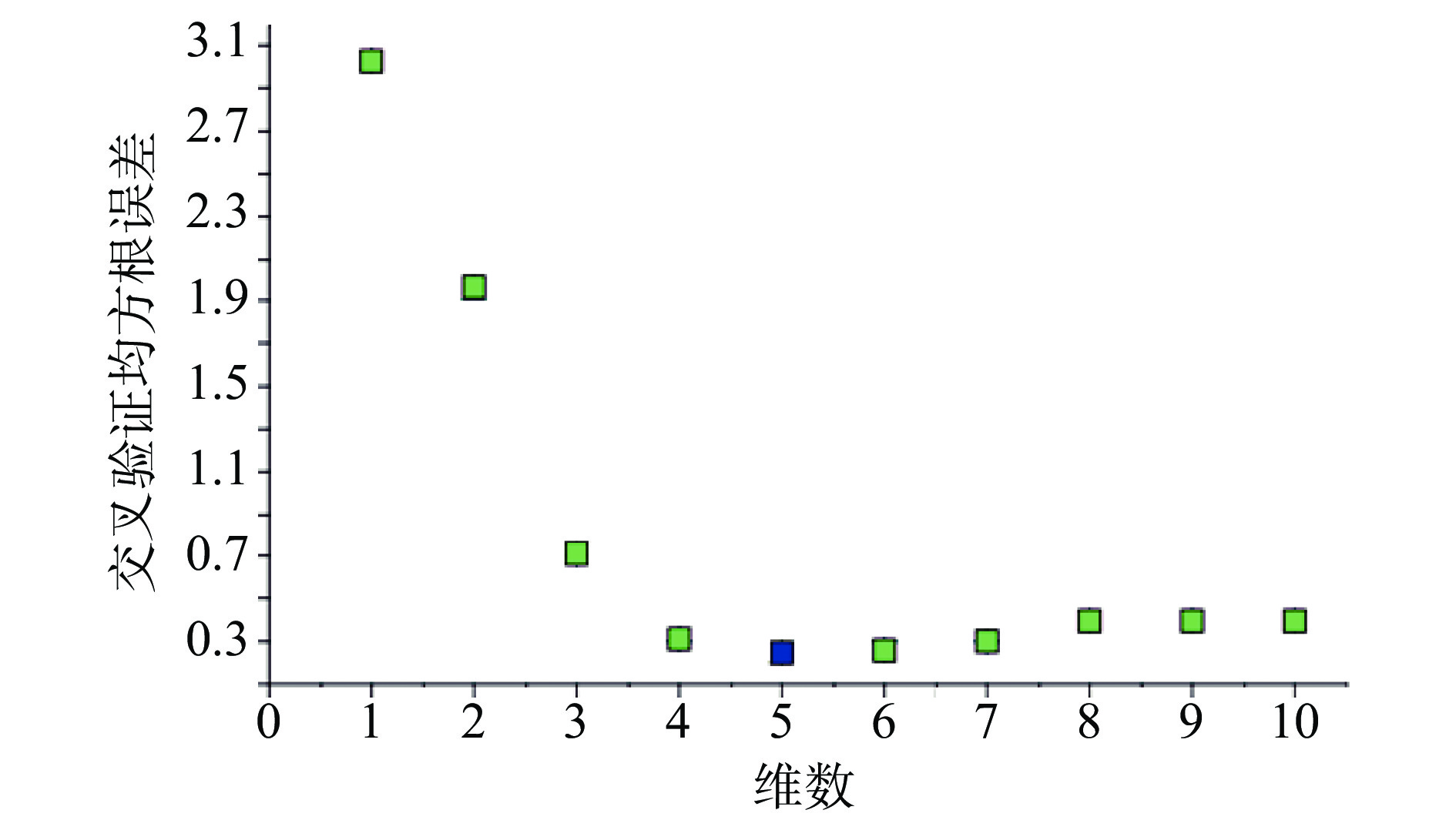
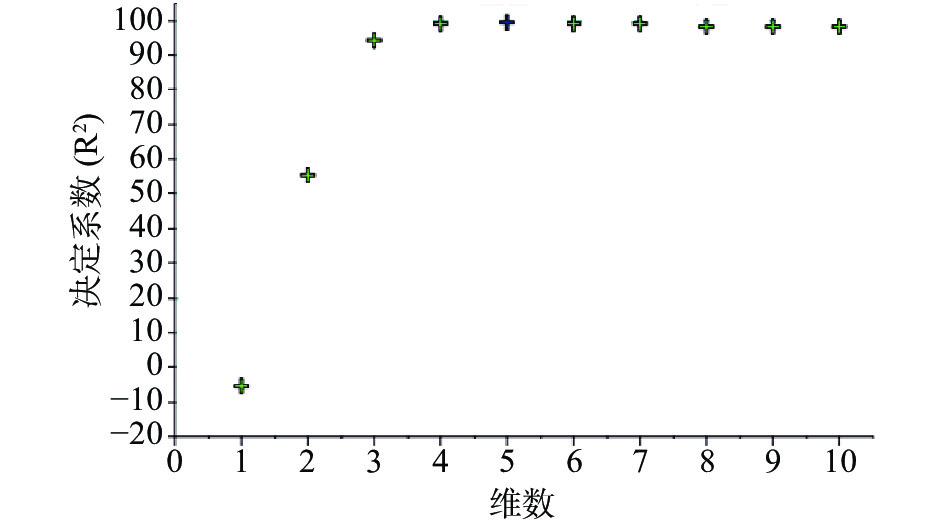
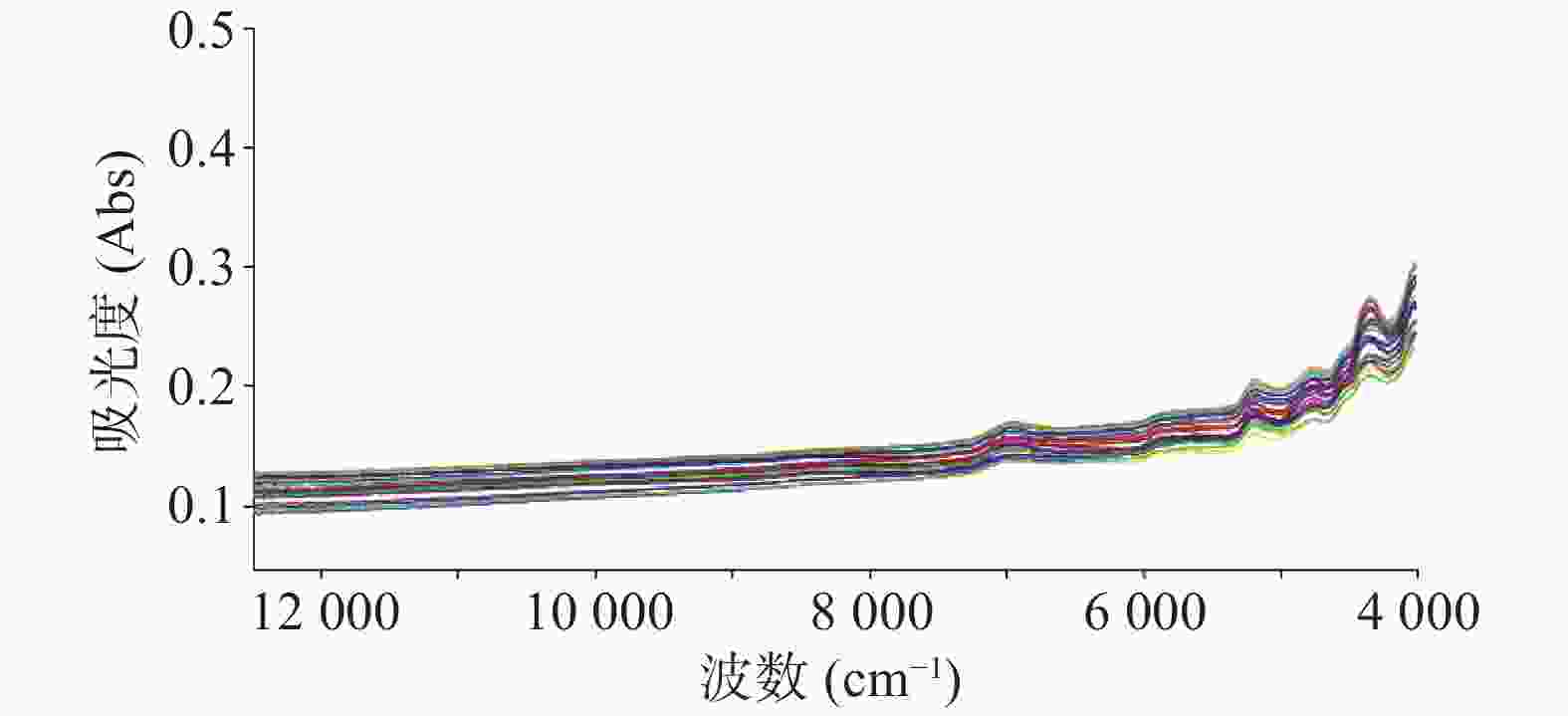
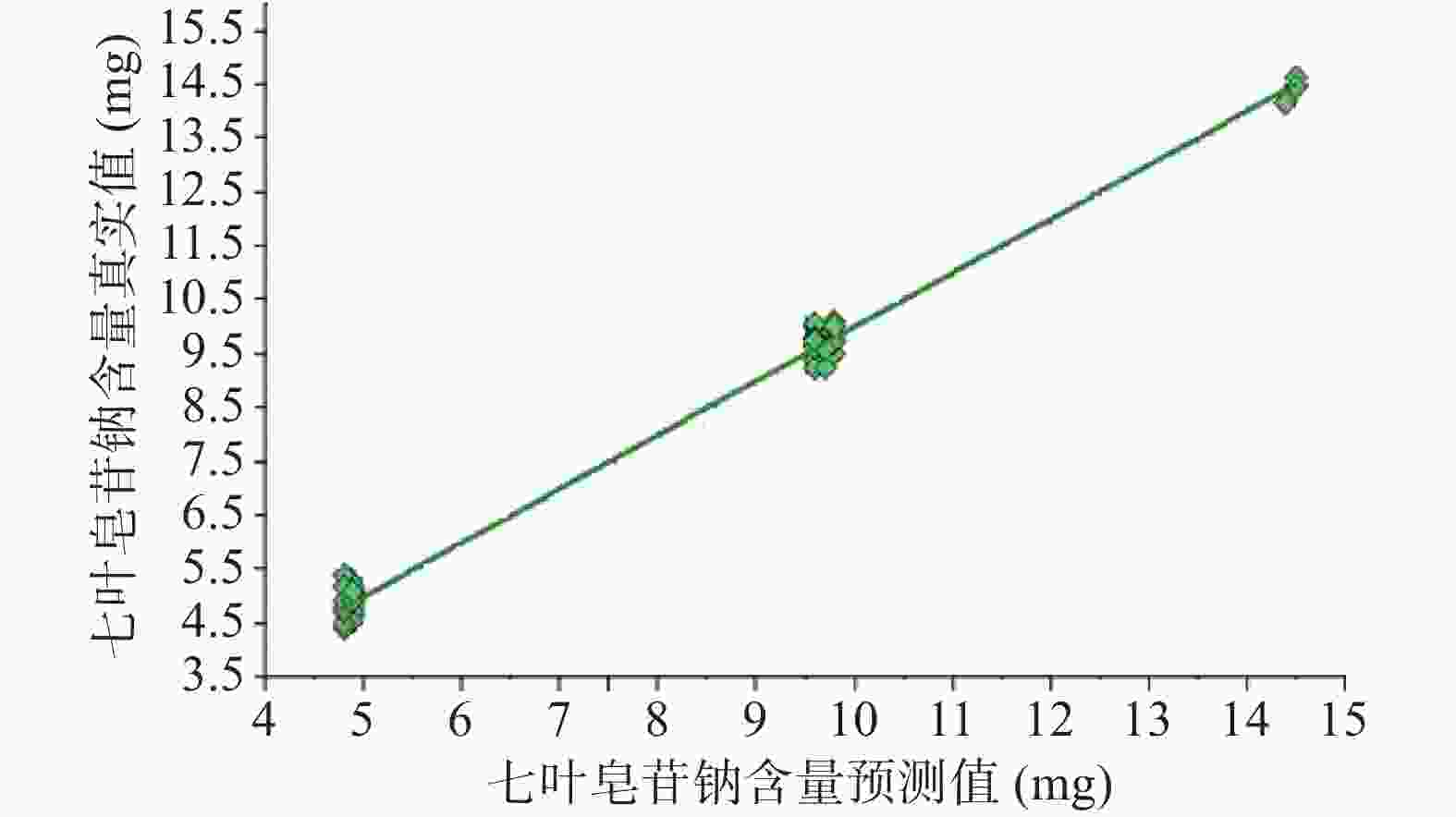
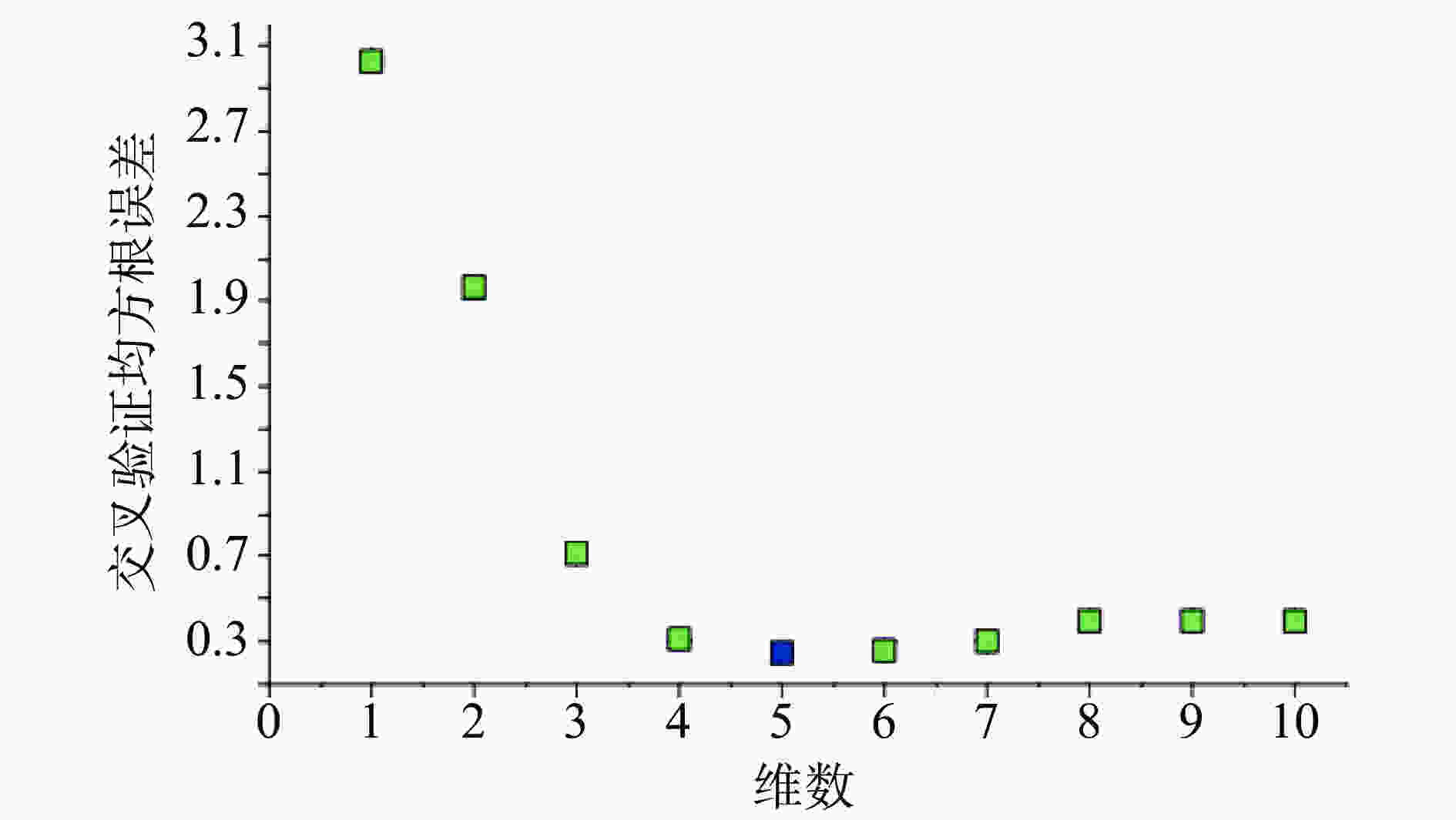
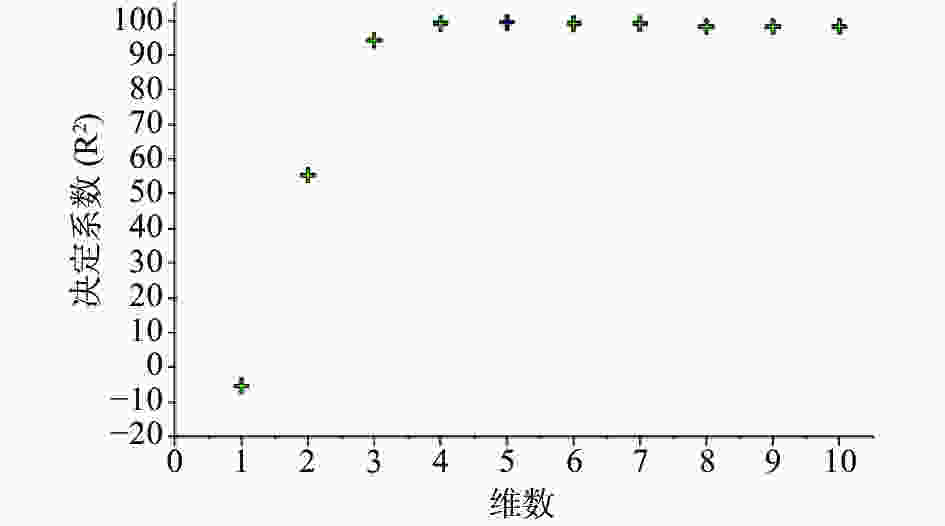
注射用七叶皂苷钠定量模型的近红外光谱图详见图2,内部验证的校正曲线见图3,RMSECV/维数见图4,R2/维数走势见图5。结果显示,注射用七叶皂苷钠定量模型R2值达到0.9926,RMSECV为0.253。在预测试验中,样品的预测值与酸碱滴定法测定值偏差均小于5%(详见表3),表明该模型能够准确预测七叶皂苷钠的含量。
样品编号 酸碱滴定法测定值(%) 预测值(%) 偏差(%) 样品编号 酸碱滴定法测定值(%) 预测值(%) 偏差(%) 1 93.2 95.4 2.2 20 95.8 95.9 0.1 2 95.8 96.8 1.0 21 97.6 96.6 −1 3 97.9 96.6 −1.3 22 97.5 98.4 0.9 4 96.2 96.1 −0.1 23 97.1 96.6 −0.5 5 96.9 95.8 −1.1 24 96.4 96.5 0.1 6 96.4 96.8 0.4 25 96.9 96.7 −0.2 7 95.9 96.2 0.3 26 97.3 97.0 −0.3 8 96.8 96.7 −0.1 27 96.7 97.0 0.3 9 97.1 97.3 0.2 28 97.2 97.1 −0.1 10 96.6 96.7 0.1 29 96.9 97.2 0.3 11 96.6 96.4 −0.2 30 94.4 94.5 0.1 12 97.1 97.3 0.2 31 96.5 96.2 −0.3 13 95.9 95.9 0 32 96.8 96.9 0.1 14 97.1 97.5 0.4 33 97.9 97.0 −0.9 15 96.8 96.8 0 34 97.5 96.8 −0.7 16 95.7 96.4 0.7 35 90.8 95.2 4.4 17 96.3 96.0 −0.3 36 90.1 95.0 4.9 18 96.4 96.4 0 37 96.1 96.5 0.4 19 97.5 96.6 −0.9 38 98.0 96.7 −1.3 利用已建立的定量模型,对未参与建模的5批次样品进行外部交叉验证,并将结果转化为标示量百分比后与测定值比较,偏差均小于1.5%(详见表4),表明预测值和参考值相关性良好,所建定量模型稳定可靠,可用于样品中七叶皂苷钠含量的快速检测。
样品编号 酸碱滴定法测定值(%) 预测值(%) 偏差(%) 39 95.9 96.7 0.7 40 96.8 97.5 0.7 41 96.2 97.4 1.2 42 97.6 98.0 0.4 43 96.6 97.3 0.7
3.1. 测定方法
3.2. 定性模型的建立
3.2.1. 建模图谱
3.2.2. 建模方法
3.2.3. 建模结果
3.3. 定量模型建立
3.3.1. 建模样品
3.3.2. 建模方法
3.3.3. 建模结果
-
近红外定性分析主要用于物质的判别和归类,扫描收集系列样品的近红外光谱,可组成一个多维变量空间,同类物质在该空间内处于相近位置,以此完成建模。通过调用相应模型数据,与待测样品近红外光谱进行比较评估,即可判定待测样品类别。本试验采集图谱,经一阶导数和矢量归一化(SUV)处理,可有效消除样品的基线偏移,降低背景及噪声干扰,提高信噪比,增强光谱特征;建立的注射用七叶皂苷钠定量模型,可准确预测目标成分含量,为药品快检筛查提供数据参考;定性模型,可有效区分未参与建模的其他品种样品,可用于同厂家注射用七叶皂苷钠的定性检测。与七叶皂苷钠的其他含量测定方法相比,本研究建立近红外光谱分析法模型专属性较好,具有高效、无损、操作简单、结果准确等特点,能满足在药品质量安全监管工作中,开展批量样品现场快检的要求。在对不同厂家的注射用七叶皂苷钠进行的含量预测时发现,因不同厂家样品使用的辅料、西林瓶、填装样品厚度均存在较大差异,致使不同厂家预测结果与实际测量值偏差较大,预测值与容量分析法含量测定结果偏差可达47.0%。因此,本实验所建定量模型仅适用于同厂家样品含量预测,在实际应用中,可针对不同厂家建立系列定量模型,用于该药品的快速鉴定检测。







 DownLoad:
DownLoad: