-
口腔溃疡膜是海军军医大学附属长征医院的特色院内制剂,该制剂主要由克霉唑、硫酸新霉素、冰片、盐酸达克罗宁、地塞米松磷酸钠等成分组成,呈微黄色至淡黄色半透明膜。临床用于治疗多发性口腔溃疡。溃疡是口腔疾病的常见表现,口腔黏膜溃疡病因可分为4类:感染性、免疫性、外伤性和肿瘤性[1]。地塞米松磷酸钠属糖皮质激素,经常用于加速溃疡愈合、减轻口腔溃疡的局部症状[2];克霉唑是一种常用于治疗真菌感染的抗真菌药物[3]。口腔溃疡膜能保护局部溃疡面,延长局部作用时间、提高药物浓度,具有定位释放、使用方便等优点[4]。本制剂室唐蕾等[5]通过测定口腔溃疡膜中克霉唑和地塞米松磷酸钠的含量建立了一套操作简单、准确性好的HPLC含量测定方法,适用于口腔溃疡膜的质量标准控制。但随着制剂标准的提高,在《军队医疗机构制剂许可证》验收细则中明确规定:配制制剂的原料药应符合法定药品标准,辅料及直接接触制剂的包装材料应符合法定标准。因此,一些原料药及辅料在更换药用标准后,按照原先的样品前处理方法测得的含量一直偏低。本文以口腔溃疡膜中主要有效成分地塞米松磷酸钠和克霉唑的含量为指标,对口腔溃疡膜中有效成分的提取工艺进行研究,并进一步优化地塞米松磷酸钠和克霉唑的含量测定方法。
HTML
-
Agilent 1260型高效液相色谱仪(美国Agilent公司):包含G7104C(四元泵)输液泵,G7129C自动进样器,G7116A柱温箱,G7115A DAD检测器;色谱柱为Agilent Zobax SB-C18柱(250 mm×4.6 mm,5 μm,美国Agilent公司);Sartorius 天平(型号:BSA124S-CW,十万分之一天平,赛多利斯科学仪器有限公司);超声仪(SK7200H型,上海科导超声仪器有限公司);Eppendorf移液器(德国);WL-901 Vortex旋涡振荡混和器(海门市其林贝尔仪器制造有限公司)。
-
地塞米松磷酸钠对照品(批号:ZN1107XA)、克霉唑对照品(批号:H01S9Z68980),购自上海源叶生物科技有限公司;甲醇(色谱纯,德国Fisher公司);娃哈哈纯净水(纯净水,杭州娃哈哈集团有限公司);磷酸二氢钾等其余试剂均为分析纯。口腔溃疡膜(批号:190919、190923、190926,每片含地塞米松磷酸钠0.4 mg,克霉唑6.53 mg,海军军医大学附属长征医院制剂中心自制)。
1.1. 仪器
1.2. 试剂与药品
-
色谱柱:Agilent Zobax SB-C18柱(250 mm×4.6 mm,5 μm);流动相:甲醇−0.05 mol/L磷酸二氢钾溶液(70∶30, 用磷酸溶液调pH值4.4~4.5);紫外检测波长:230 nm;流速:1.0 ml/min;柱温:30 ℃;采集时间:30 min,进样量:20 μl。
-
精密称取地塞米松磷酸钠16.328 mg置于10 ml量瓶中,加甲醇-水(7∶3)溶解并稀释至刻度,摇匀,得浓度为1.632 8 mg/ml的地塞米松磷酸钠对照品储备液。精密称取克霉唑对照品20.058 mg置于10 ml量瓶中,加甲醇-水(7∶3)溶解并稀释至刻度,摇匀,得浓度为2.005 8 mg/ml的克霉唑对照品储备液。
-
分别精密量取地塞米松磷酸钠、克霉唑对照品储备液适量,加甲醇-水(7∶3)配制成含地塞米松磷酸钠16.328 μg/ml和克霉唑200.58 μg/ml的混合对照品溶液。
-
取口腔溃疡膜剪成3~5 mm大小的碎片,精密称取样品0.2 g,置于100 ml容量瓶中,加入30 ml水,超声30 min,取出,放冷,加入甲醇并稀释至刻度,摇匀,即得。
-
参照本课题组前期实验方法,制备阴性对照品溶液[5]。
-
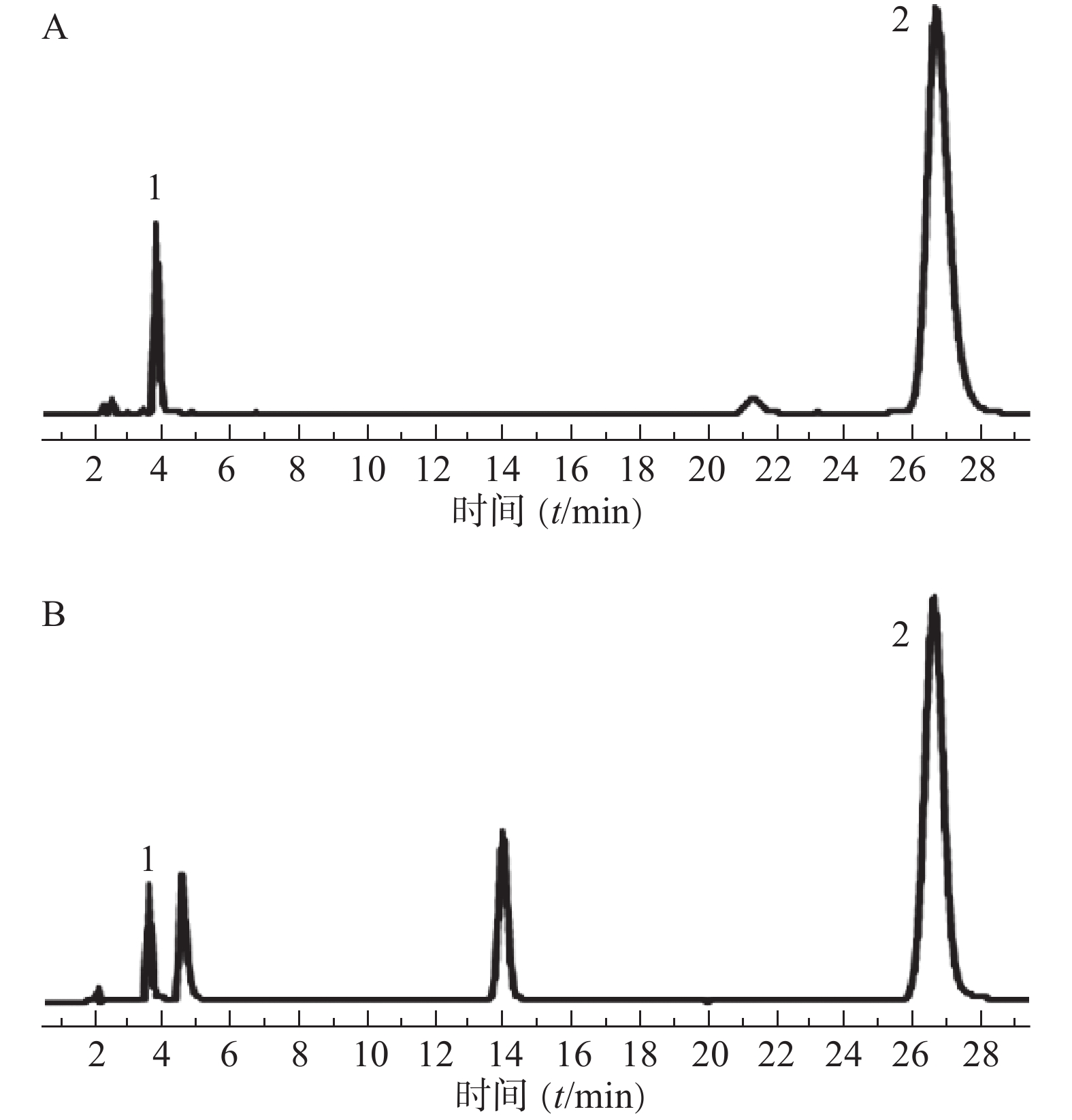
分别取混合对照品溶液(地塞米松磷酸钠、克霉唑)和供试品溶液,按“2.1”项下色谱条件测定,记录色谱图,结果见图1。图中显示供试品溶液中地塞米松磷酸钠和克霉唑保留时间与对照品溶液相应色谱峰的保留时间一致。地塞米松磷酸钠和克霉唑与其相近的其他峰分离完全(分离度>1.5),即本试验条件下地塞米松磷酸钠和克霉唑与其他组分分离完全。
-
精密量取“2.2.2”项下混合对照品溶液适量,稀释成以下浓度的混合对照品溶液:地塞米松磷酸钠0.482 0、0.816 4、1.632 8、4.082 0、8.164 0、16.328 0 μg/ml;克霉唑5.014 5、10.029 0、20.058 0、50.14 50、100.290 0、200.580 0 μg/ml,按“2.1”项下色谱条件进行测定,并记录色谱峰面积。以峰面积为纵坐标(Y),对照品质量浓度为横坐标(C),绘制标准曲线,并进行线性回归,结果见表1。
成分 回归方程 r 线性范围(μg/ml) 地塞米松磷酸钠 Y=34.961C+27.986 0.999 9 0.482 0~16.328 0 克霉唑 Y=32.366C+24.092 0.999 8 5.014 5~200.580 0 -
取同一批口腔溃疡膜(批号:190919),按“2.2.3”项方法制备供试品溶液,按“2.1”项色谱条件进行测定,连续进样6次,记录峰面积。计算地塞米松磷酸钠和克霉唑峰面积RSD分别为0.65%和0.33%,表明仪器精密度良好。
-
取同一批口腔溃疡膜(批号:190919),按“2.2.3”项方法平行制备6份供试品溶液,按“2.1”项色谱条件进行测定,记录峰面积。计算地塞米松磷酸钠和克霉唑峰面积RSD分别为0.56%和0.54%,表明该方法重复性良好。
-
取同一批口腔溃疡膜(批号:190919),按“2.2.3”项方法制备供试品溶液,按“2.1”项色谱条件,分别在0、2、4、6、12、24 h进样测定,记录峰面积。计算地塞米松磷酸钠和克霉唑峰面积RSD值分别为0.94%和1.33%,表明供试品溶液在室温放置24 h内稳定性良好。
-
精密称取已知含量的样品(批号:190919)0.2 g,共6份,置于100 ml容量瓶中,加入各对照品适量,按“2.2.3”项方法制备供试品溶液,按“2.1”项色谱条件进样测定,记录峰面积,计算加样回收率。结果地塞米松磷酸钠和克霉唑的平均加样回收率分别为(103.97±1.02)%和(104.23±0.63)%,表明本法回收率良好。
-
取3批口腔溃疡膜,按“2.2.3”项方法制备供试品溶液,按“2.1”项色谱条件进行测定,计算样品中地塞米松磷酸钠和克霉唑的含量,结果见表2。
批号 含量[标示量(%)] 地塞米松磷酸钠 克霉唑 190919 76.600 0±0.005 2 86.800 0±0.011 3 190923 85.900 0±0.011 5 99.100 0±0.025 9 190926 74.900 0±0.043 7 85.700 0 ±0.067 6
2.1. 色谱条件
2.2. 溶液的制备
2.2.1. 对照品溶液
2.2.2. 混合对照品溶液
2.2.3. 供试品溶液
2.2.4. 阴性对照品溶液
2.3. 方法学考察
2.3.1. 专属性试验
2.3.2. 线性关系的考察
2.3.3. 精密度试验
2.3.4. 重复性试验
2.3.5. 稳定性试验
2.3.6. 加样回收率试验
2.3.7. 样品含量测定
-
本研究根据口腔溃疡膜的特性,对溶剂种类、溶剂提取顺序进行考察,由于口腔溃疡膜中含羧甲基纤维素钠,这是由一种天然纤维素衍生的阴离子水溶性聚合物[6],易溶于水,因此需要一定比例的水将膜剂破坏,才能释放出膜剂中的有效成分地塞米松磷酸钠和克霉唑。
-
取口腔溃疡膜剪成3~5 mm大小的碎片,精密称取样品0.2 g,置于100 ml容量瓶中,加入甲醇35 ml,超声30 min,取出,放冷,加入15 ml水,混匀后用甲醇-水(7∶3)定容,摇匀,即得。
-
取口腔溃疡膜剪成3~5 mm大小的碎片,精密称取样品0.2 g,置于100 ml容量瓶中,加入30 ml水,超声30 min,取出,放冷,加入甲醇并稀释至刻度,摇匀,即得。
-
取口腔溃疡膜剪成3~5 mm大小的碎片,精密称取样品0.2 g,置于100 ml容量瓶中,加入甲醇-水(7∶3)30 ml,超声30 min,取出,放冷,加入甲醇-水(7∶3)稀释至刻度,摇匀,即得。
用以上不同溶剂提取口腔溃疡膜,每份样品、每种溶剂平行提取3次,按“2.1”项所述色谱条件测定地塞米松磷酸钠和克霉唑含量,结果见表3。实验结果显示:水超声提取和70%甲醇超声提取对口腔溃疡膜中有效成分的提取效果相当,但水超声提取相对简单,故选用水超声提取30 min为最佳提取工艺。
-
在液相色谱条件优化实验中,比较了不同pH时的采集时间,结果发现:pH在4.4~4.5时采集时间为30 min,较之于前缩短了5 min,方法更便捷。
提取方法 地塞米松磷酸钠 克霉唑 甲醇超声提取 67.46 74.49 71.58 79.06 73.09 72.64 水超声提取 108.29 101.28 104.08 101.03 105.88 103.38 70%甲醇超声提取 104.27 95.00 109.52 100.52 106.89 103.54 本研究基于HPLC同时测定口腔溃疡膜中地塞米松磷酸钠、克霉唑含量的方法,经提取工艺的优化和全面的方法学考察,成功用于测定样品中地塞米松磷酸钠和克霉唑的含量,该法简便可靠,可为口腔溃疡膜的质量控制提供依据,也为其质量标准研究奠定了基础。








 DownLoad:
DownLoad: