-
骨质疏松症(osteoporosis, OP)是以骨微细结构破坏和骨量减少为特征的全身性骨代谢疾病,可导致骨脆性和骨折风险增加,多见于老年人。衰老是致骨质疏松的一个重要因素,随着机体的衰老,骨骼中过多的活性氧(reactive oxygen species, ROS)抑制成骨细胞的增殖、分化和成熟,抑制成骨细胞分泌骨基质及骨基质的矿化,同时促进破骨细胞骨吸收,导致骨质疏松的发生。2018年国家卫生健康委员会发布OP流行病学调查结果显示,我国65岁以上人群OP患病率达32.0%,其中,男性为10.7%,女性为51.6%[1]。四烯甲萘醌(menatetrenone, MK4)在临床上常单独或协同用于防治老年性骨质疏松症,疗效显著[2-3],是我国现版《原发性骨质疏松症诊疗指南》和《骨质疏松症中西医结合诊疗指南》的推荐药物[1-2]。药理研究发现,MK4能促进成骨增殖、分化和碱性磷酸酶(alkaline phosphatase, ALP)活性[3-4]。MK4能通过调节氧化应激相关基因和蛋白,在成骨细胞骨形成中发挥保护作用[5],且能阻止成骨细胞凋亡[6]。过氧化氢(H2O2)是ROS在体内存在的主要形式,会穿透成骨细胞造成细胞损伤,本研究拟探讨MK4对H2O2刺激成骨细胞氧化损伤的保护作用和调控机制,阐明MK4抗老年性骨质疏松的作用机制。
HTML
-
成骨细胞系MC3T3-El(中国科学院上海生命科学研究细胞资源中心);特级胎牛血清(FBS)、DMEM高糖培养基、胰蛋白酶、双抗(青霉素和链霉素混合液)和PBS缓冲液(pH=7.2)均购自美国GIBCO公司;噻唑蓝(MTT)、MK4(Sigma公司)。过氧化氢(H2O2,比利时Acros Organics公司);丙二醛(MDA)试剂盒、谷胱甘肽(GSH)试剂盒、超氧化物歧化酶(SOD)试剂盒、JC-1线粒体膜电势(MMP)试剂盒和活性氧(ROS)试剂盒均购自上海碧云天生物技术有限公司;BCA蛋白检测试剂盒、Annexin V-FITC/PI凋亡检测试剂盒、核酸提取试剂盒、反转录试剂盒和扩增试剂盒(均为Thermo Fisher公司产品);叉头框蛋白(FoxO1和FoxO3)、β细胞淋巴瘤/白血病基因2(Bcl2)和凋亡基因(Bax)等引物购自生工生物工程(上海)股份有限公司。
-
复苏MC3T3-El小鼠成骨细胞系,置于5 ml含10% FBS的DMEM培养基中,放入37 ℃、5% CO2培养箱。以2×104/ml浓度的成骨细胞接种于96孔培养板中培养24 h后,分别采用0、10、20、50和100 μmol/L(n =10)的H2O2处理,培养4、12、24 h后,采用碧云天MTT试剂盒测定细胞活力。
以2×104/ml浓度的成骨细胞接种于96孔培养板中培养24 h后,并按空白、氧化应激模型、药物剂量分组:①对照组,②选择合适浓度H2O2组,③H2O2 +10 μmol/L MK4组,④H2O2 +1 μmol/L MK4组,⑤H2O2 + 0.1 μmol/L MK4组。加入药物培养24 h,采用MTT法检测细胞增殖活性。
-
以2×104/ml浓度的成骨细胞接种于96孔培养板中培养24 h,按照方法“2.1”项下设置各实验组,连续培养6 d,每3 d换液1次,采用硝基苯酚磷酸二钠法检测ALP活性。给药6 d后,弃培养液,PBS洗3次,依次加入100 μl二乙醇胺(50 mmol/L),50 μl的对硝基苯酚磷酸二钠(2.5 mmol/L),在37 ℃孵育30 min,再加入50 μl的0.3 mol/L氢氧化钠溶液终止反应,置405nm处,测吸光度值(A)。以不同浓度的对硝基苯酚溶液绘制标准曲线,ALP活性由每孔释放的对硝基苯酚的μmol数表示。
-
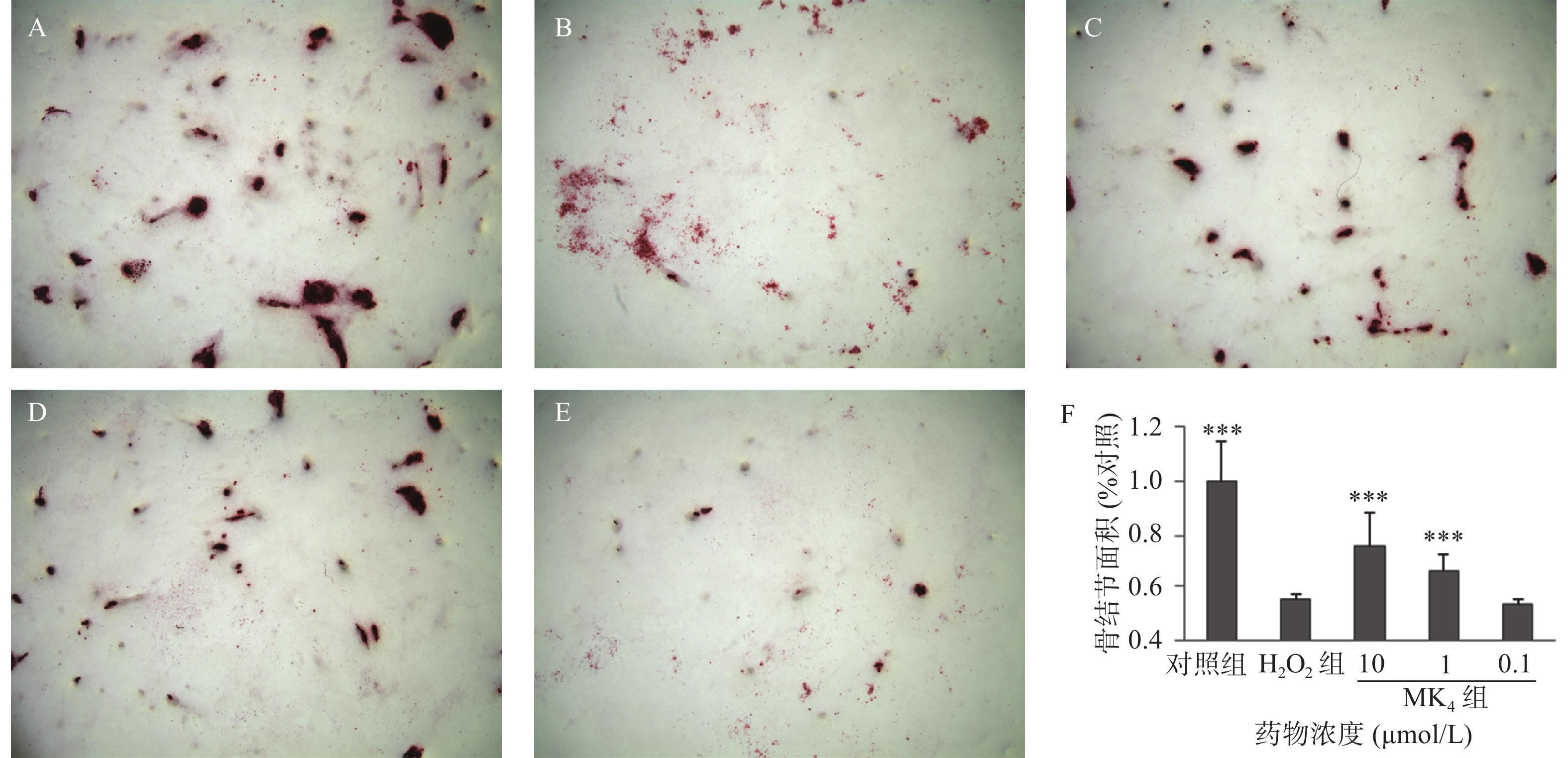
以5×104/ml浓度的成骨细胞将MC3T3-El细胞接种于12孔板内,放入37 ℃,5% CO2培养箱,12 h后换骨结节诱导培养基(0.1%牛血清白蛋白、10 nmol/L地塞米松、10 mmol/L β-甘油磷酸钠、50 μg/ml抗坏血酸以及10%胎牛血清的DMEM培养基)培养24 h,按照方法“2.1”项下设置各实验组,每3 d换液1次,连续培养14 d,采用0.1%茜素红-Tris-Hcl染液(pH 8.3)染色,37 ℃下染色30 min,采用倒置相差显微镜(Leica DMI 3000)观察,并随机拍照10张,用image-Pro Plus (IPP 6.0)分析骨结节面积。
-
以2×105/ml细胞浓度铺6孔板,培养24 h后,按照方法“2.1”项下设置各实验组,干预24 h后,用荧光酶标仪法分别测定JC-1单体和复合物的荧光,DCFH-DA探针法测活性氧水平,ELISA法测定GSH、SOD和MDA水平。
-
以1×106/ml细胞浓度铺6孔板,培养12 h后,按照方法“2.1”项下设置各实验组,干预24 h后,依据Annexin V-FITC/PI凋亡检测试剂盒说明书,采用流式细胞仪法检测。
-
以1×106/ml细胞浓度铺6孔板,培养12 h后,按照方法“2.1”项下设置各实验组,干预24 h后,依据试剂盒说明书进行提取总RNA和反转录后,分别对GAPDH、SOD2、FoxO1、FoxO3、Bcl-2和bax进行RT-PCR扩增,引物序列见表1,PCR反应条件:采用预变性95 ℃、10 min,变性95 ℃、45 s,退火60 ℃、45 s,延伸72 ℃、50 s,循环40次,总反应体系为10 μl。
基因 上游引物 下游引物 SOD2 TCCCAGACCTGCCTTACGA TCGGTGGCGTTGAGATTG FoxO1 GTACGCCGACCTCATCACCAAG GCACGCTCTTCACCATCCACTC FoxO3 TGCTAAGCAGGCCTCATCTCAA AAGCTGTAAACGGATCACTGTC Bcl-2 AGGAGCAGGTGCCTACAAGA GCATTTTCCCACCACTGTCG bax CATCCAGGATCGAGCAGA GCCTTGAGCACCAGTTTG GAPDH TGAACGGGAAGCTAAGG TCCACCACCCTGTTGCTGGA -
每组实验重复3次。采用SPSS软件经ANOVA方差分析检验,差异有统计学意义(α=0.05),再采用Student's t test检验进行两组比较,以P<0.05为差异有统计学意义。
2.1. MTT法检测成骨细胞活力
2.2. 成骨细胞ALP活性
2.3. 成骨细胞骨结节面积
2.4. 细胞线粒体膜电势、ROS和氧化应激酶MDA、GSH、SOD测定
2.5. 细胞凋亡检测
2.6. RT-PCR
2.7. 统计学分析
-
MC3T3-E1经0~100 μmol/L H2O2分别处理4、12、24 h,结果发现0~100 μmol/L H2O2处理4 h对细胞活力均无显著性影响(P>0.05),处理12 h后,在50和100 μmol/L H2O2下的细胞活力显著降低,分别降低19.4%和33.4%。H2O2干预24 h后,在20、50、100 μmol/L均具有显著性差异,分别降低13.2%,47.4%和57.9%(表2)。故后续实验选择20 μmol/L处理24 h。
处理时间(t/h) 对照组 H2O2浓度(μmol/L) 10 20 50 100 4 0.22±0.04 0.23±0.03 0.22±0.04 0.21±0.05 0.20±0.04 12 0.31±0.05 0.33±0.04 0.30±0.04 0.27±0.02* 0.22±0.03* 24 0.38±0.03 0.39±0.03 0.34±0.02* 0.20±0.03** 0.16±0.04** *P<0.05,**P<0.01,与对照组比较。 -
MC3T3-E1经20 μmol/L H2O2处理24 h后,结果显示,显著抑制了成骨细胞的细胞活力(P<0.05),ALP活性(P<0.05)和骨结节形成面积(P<0.05),与空白组比较,分别降低13%、16%和85%,见表3和图1。与模型组比较,MK4在1~10 μmol/L可促进H2O2损伤成骨细胞增殖(P<0.05)。同样,MK4在1~10 μmol/L能显著改善ALP活性(P<0.05)和提高骨结节形成面积(P<0.05),分别增加50.7%和44.5% (表3和图1)。
组别 MTT(%对照) ALP(%对照) MPP(%对照) 活性氧(%对照) MDA(μmol/g) 细胞凋亡率(%) 对照组 1.00±0.01* 1.00±0.02* 1.00±0.02* 1.00±0.01* 27.2±4.3** 2.3±0.3* H2O2组 0.87±0.02 0.84±0.03 0.78±0.05 1.13±0.02 51.0±3.7 7.3±0.3 H2O2+MK4(10 μmol/L)组 1.03±0.04* 0.99±0.03* 1.05±0.07* 1.02±0.02* 27.3±3.1** 1.8±0.2* H2O2+MK4(1 μmol/L)组 0.96±0.03 0.87±0.04 0.88±0.21 1.06±0.05 44.8±2.0 2.9±0.3* H2O2+MK4(0.1 μmol/L)组 0.85±0.03 0.80±0.03 0.82±0.09 1.09±0.05 51.6±0.3 5.7±0.4 *P<0.05,**P<0.01,与H2O2组比较。 -
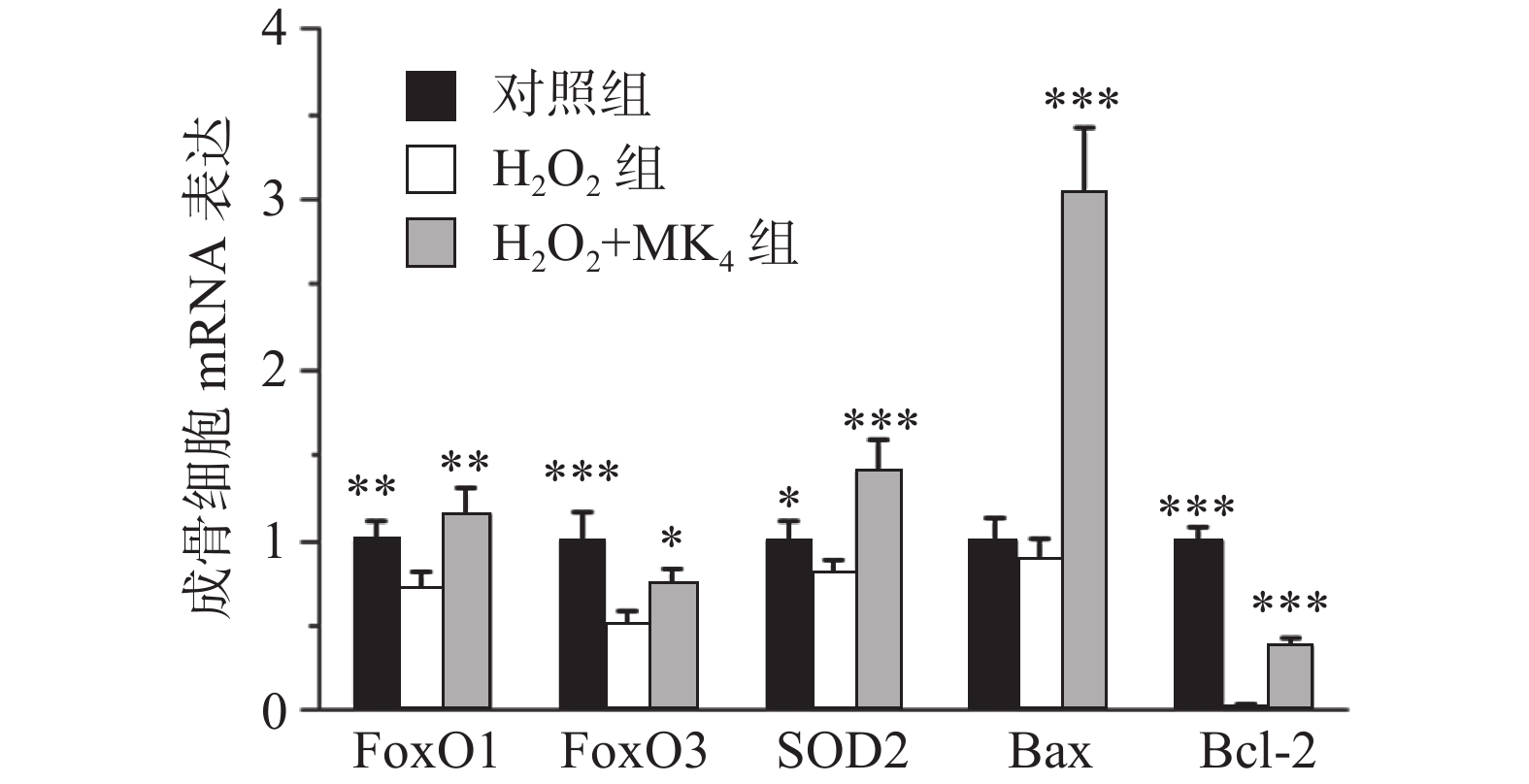
MC3T3-E1成骨细胞经20 μmol/L H2O2处理24 h后,结果发现显著降低成骨细胞膜电势和增高活性氧含量(P<0.05)。与模型组比较,MK4在10 μmol/L可促进H2O2损伤成骨细胞膜电势升高和降低活性氧含量(P<0.05)。与空白组比较,20 μmol/L H2O2能显著降低FoxO1, FoxO3和SOD2的mRNA表达(P<0.05)。与H2O2组比较,给予10 μmol/L MK4后,FoxO1, FoxO3和SOD2的mRNA表达均显著增高(P<0.01)。
-
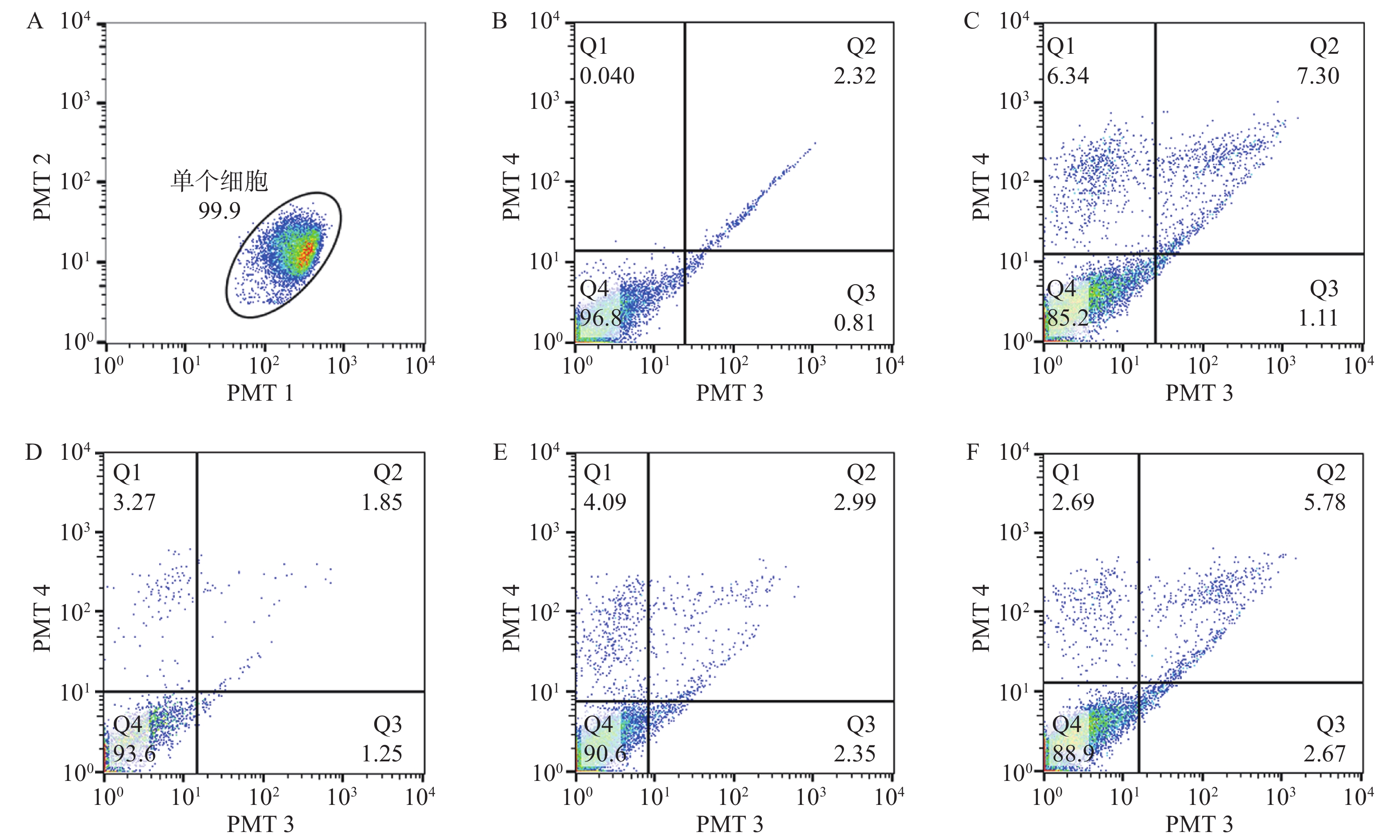
在通道1和通道2观察单个细胞分布区域,选定99.9%的细胞区域用于后续分析,在通道3和通道4观察凋亡细胞分布,Q4为正常细胞,Q1为坏死细胞;Q2为晚期凋亡细胞,Q3为早期凋亡细胞。与对照组比较,20 μmol/L H2O2处理24 h后,细胞的凋亡率显著增高(P<0.05)。经0.1~10 μmol/L浓度MK4干预24 h后发现,1~10 μmol/L浓度MK4能显著降低细胞凋亡率(P<0.05),与对照组相近,见表3和图2。与空白组比较,20 μmol/LH2O2能显著降低Bcl-2的mRNA表达(P<0.001),bax表达无显著差异,给予10 μmol/L MK4后,Bcl-2和bax的mRNA表达均显著增高(P<0.01),见图3。H2O2组的bax/Bcl-2比值为对照组的22.5倍,而MK4处理后降低至对照组的7.6倍。
3.1. H2O2处理MC3T3-El成骨细胞
3.2. MK4对H2O2损伤成骨细胞增殖、ALP活性和骨结节形成的影响
3.3. MK4对H2O2损伤成骨细胞线粒体膜电势、活性氧和抗氧化酶的影响
3.4. MK4对H2O2损伤成骨细胞凋亡的影响
-
骨代谢中,机体通过调节FoxOs转录因子的活性,产生抗氧化物酶,对抗氧化应激对骨骼的损伤,包括FoxO1、FoxO3、FoxO4和FoxO6等,其中,FoxO1和FoxO3是调节成骨细胞氧化还原平衡和成骨功能的主要分子[7]。活性氧可激活FoxO1的转录,调节线粒体抗氧化酶Mn-SOD的活性[8]。随着活性氧的升高,FoxO3下调,成骨细胞分化受损,抑制骨形成作用[9]。本研究发现,MK4对H2O2引起的氧化应激具有显著的改善作用,降低活性氧和脂质氧化产物MDA水平,上调转录因子FoxO1、FoxO3和抗氧化酶SOD的mRNA表达。
Bcl-2蛋白可减少氧化应激水平,而bax基因可与Bcl-2形成异源二聚体,抑制Bcl-2的作用,进而诱导细胞凋亡。MK4可上调Bcl-2/bax比值,抑制了成骨细胞凋亡[10]。本研究发现,H2O2能显著提高成骨细胞凋亡率,同时增加bax的表达,降低Bcl-2蛋白表达。MK4处理组对成骨细胞凋亡的拮抗作用明显,随着剂量浓度增加而增加。MK4在在氧化应激状态下,可显著上调Bcl-2,下调bax的基因表达,bax/Bcl-2比值显著降低,抑制了成骨细胞凋亡。
FoxOs激活促进Bcl-2相关凋亡调节蛋白(Bim)转录,Bim是线粒体凋亡通路的核心调控者,引起成骨细胞线粒体膜电位降低[10]。线粒体跨膜电位降低说明线粒体膜通透性转运孔(MPTP)过度开放。若MPTP过度开放,易引起呼吸链解偶联,线粒体基质渗透压增高,使得促凋亡活性物质从线粒体释放入细胞基质,导致细胞凋亡。MK4显著改善了H2O2刺激的成骨细胞线粒体膜电势降低,表明MK4对H2O2刺激成骨细胞的氧化损伤具有保护作用。
综上所述,MK4能显著抑制H2O2刺激的成骨细胞氧化损伤,机制与FoxO转录因子相关。同时,MK4对H2O2引起的成骨细胞凋亡具有拮抗作用,其机制为上调Bcl-2和下调bax的基因表达。









 DownLoad:
DownLoad: