-
抗菌药物耐药性是一个全球性的公共卫生问题。碳青霉烯类抗生素被认为是治疗多重耐药菌感染的最后防线,但随着其在临床的广泛应用及不合理使用,导致耐碳青霉烯类肠杆菌(CRE)特别是耐碳青霉烯类肺炎克雷伯菌(CRKP)激增,给临床抗感染治疗带来巨大挑战。
头孢他啶/阿维巴坦(ceftazidime/avibactam,CAZ/AVI)是一种新型β-内酰胺类/β-内酰胺酶抑制剂,研究表明,阿维巴坦在体外可抑制Ambler分类中的A类、C类和某些D类酶的活性,由于缺乏活性位点丝氨酸残基,对B类金属酶没有活性[1]。阿维巴坦可重新环化恢复活性从而可长效抑制β-内酰胺酶[2],能够恢复或增强头孢他啶抗菌活性,CAZ/AVI组合具有广谱抗菌活性,对包括CRE在内的多重耐药革兰阴性杆菌(MDR-GNB)具有强有力的杀菌活性。CAZ/AVI于2015年在美国获批上市,随后在欧洲和中国获批上市。现已陆续被美国食品药品管理局(FDA)、欧洲药品管理局(EMA)和国家药品监督管理局(NMPA)批准用于成人治疗方案有限或无其他选择的G-菌感染的治疗。目前,有一些小样本的回顾性研究表明[3-7],CAZ/AVI可能是治疗CRE/CRKP的有效替代品,但没有关于CAZ/AVI与其他抗菌药物用于CRE/CRKP的疗效和安全性比较的系统评价,以至于无法客观的评估两者之间的差异。本研究拟采用Meta分析方法,对国内外公开发表的CAZ/AVI治疗碳青霉烯类耐药菌感染的疗效和安全性研究进行系统评价,以期为CAZ/AVI的临床选用提供循证依据。
HTML
-
计算机检索PubMed、Embase、Cochrane Library、中国生物医学文献数据库(CBM)、中国知网(CNKI)、维普中文科技期刊数据库(VIP),并在此基础上进行手工检索及追查纳入文献的参考文献。检索时限为各数据库建库起至2021年2月。中文检索词为:头孢他啶阿维巴坦;英文检索词为:“ceftazidime/avibactam”“carbapenem-resistanece Enterobacteriaceae(CRE)”“carbapenem-resistance Klebsiella pneumonia(CRKP)”“carbapenemase-producing Enterobacteriaceae(CPE)”。检索采取主题词和自由词相结合的方式检索,如PubMed检索式:(“avibactam, ceftazidime drug combination [Supplementary Concept]”OR“avibactam-ceftazidime”OR“ceftazidime-avibactam”OR“Avycaz”) AND (“carbapenem-resistanece enterobacteriaceae”OR“carbapenem-resistance klebsiella pneumonia”OR“carbapenemase-producing enterobacteriaceae”)。
-
①研究类型:收集所有类型的中、英文临床研究,包括随机对照研究、队列研究、病例对照研究、有对照的病例报告(样本量≥10)。②研究对象:年龄≥18岁,临床确诊为CRE/CRKP感染患者,性别不限。③干预措施:试验组患者接受CAZ/AVI抗感染治疗,给药时间≥24 h,用法用量及疗程不等;对照组为其他常规抗菌药物,如多粘菌素或替加环素或碳青霉烯类或β-内酰胺酶抑制剂或氨基糖苷类等抗感染治疗。④结局指标:临床治愈率、临床缓解率、28 d/30 d全因病死率、感染复发率、不良事件发生率(adverse events,AEs)和严重不良事件发生率(SAEs)。
-
①重复发表或数据重复的文献;②报道信息太少、质量差及数据无法利用或错误的文献;③会议论文、病例报告和病例系列(样本量<10)、动物实验、体外实验。
-
由2名研究者通过阅读标题和摘要对检索所得文献进行初选和去重,再阅读全文并按照纳入与排除标准独立确定纳入文献。如有分歧则讨论解决,必要时提请第三方仲裁。提取数据包括第一作者及发表年限;研究设计类型;纳入人群的基本特征(分组及样本量、年龄、性别、耐药菌等);抗菌药物给药方案和结局指标。
-
由两位评价者依据纽卡斯尔-渥太华量表[8](Newcastle-Ottawa Scale,NOS)对纳入研究进行质量评价,如存在分歧则讨论决定。NOS主要包括研究人群选择、组间可比性和结果测量3个项目共8个条目,对文献质量的评价采用星级系统的半量化原则,该量表以星数代表分值,满分为9颗星。评分>6颗星为高质量研究,评分6颗星为中等质量研究,评分<6颗星为低质量研究[9]。NOS评分≥6颗星的文献方可纳入研究。
-
采用Cochrane协作网提供的RevMan 5.3统计软件进行Meta分析。本研究所有结局指标均为计数资料,故采用效应量比值比(OR)及其95%置信区间(CI)表示。采用χ2检验分析各研究结果间的统计学异质性,若各研究结果间异质性无统计学意义(P>0.10或I2<50%),则采用固定效应模型进行Meta分析,反之,则采用随机效应模型进行Meta分析。
1.1. 检索策略
1.2. 文献纳入和排除标准
1.2.1. 纳入标准
1.2.2. 排除标准
1.3. 数据提取
1.4. 质量评价
1.5. 统计学方法
-
初筛得到相关文献564篇,其中,英文文献486篇(PubMed 315篇、Embase 83篇、Cochrane Library 88篇),中文文献78篇(CBM 28篇、CNKI 27篇、VIP 23篇)。剔除重复文献后获得248篇,阅读标题后排除与CAZ/AVI治疗CRE/CRKP/CPE感染不相关文献227篇。阅读剩余文献摘要,排除结局指标缺失或指标不相关文献33篇,得到文献11篇。阅读全文剔除样本量<10的文献后,最终纳入5篇英文文献[3-7]进入本研究。共计患者392例,其中,试验组110例,对照组282例。其中,1项为前瞻性研究,4项为回顾性研究。4项研究为CRE感染,1项研究为CRKP感染,4项CRE研究进行了碳青霉烯酶检测确定绝大部分菌株产KPC酶。纳入研究的基本信息见表1。
纳入研究 中心情况 研究类型 研究时间(年) 分组及样本量 平均年龄(岁) 男性(%) 致病菌 观察时间(t/d) 给药方案 伴随疗法 结局指标 Shields[3]
2017单中心
(美国)回顾性 2009-2017 T(13) 66 54 CRKP 90 CAZ/AVI GEN ①③④⑤⑥⑦ C(96) 59 (57.3) CB+AG,CB+COL,其他疗法 Castón[4]
2017多中心(西班牙、以色列) 回顾性 2012-2016 T(8) 61 50 CRE 30 CAZ/AVI AM,CB,FOS,TGC,COL ①③ C(23) 59 (65.2) CB,AG,
BLIBL,TGC,
FOS,COLDuin[5] 2018 多中心
(美国)前瞻性 2011-2016 T(38) 57 61 CRE 30 CAZ/AVI TIG,AG,GEN,CB,FOS,
SXT①②③⑥⑦ C(99) 63 (42) COL Alraddadi[6]2019 单中心(沙特阿拉伯) 回顾性 2017-2018 T(10) 59.5 80 CRE 30 CAZ/AVI - ①②③⑤ C(28) 61.5 (57.1) COL,CB,TGC,AM,QU,SXT,AZT Tsolaki[7]
2019多中心
(希腊)回顾性 40个月 T(41) 61.9 68.3 CRE 28 CAZ/AVI - ①③⑤ C(36) 59.1 (77.3) 适当抗生素
治疗注:T:试验组;C:对照组。结局指标:①临床治愈率;②临床缓解率;③28 d/30 d全因病死率;④感染复发率;⑤AEs;⑥SAEs。CAZ/AVI:头孢他啶/阿维巴坦;CB:碳青霉烯类;AG:氨基糖苷类;GEN:庆大霉素;COL:多粘菌素E;TGC:替加环素;BLIBL:β-内酰胺酶抑制剂;FOS:磷霉素;QU:喹诺酮类;SXT:复方磺胺甲唑;AZT:氨曲南。 -
依据NOS对5项观察性研究进行质量评价,NOS评分均为6颗星,符合纳入Meta分析标准。
-
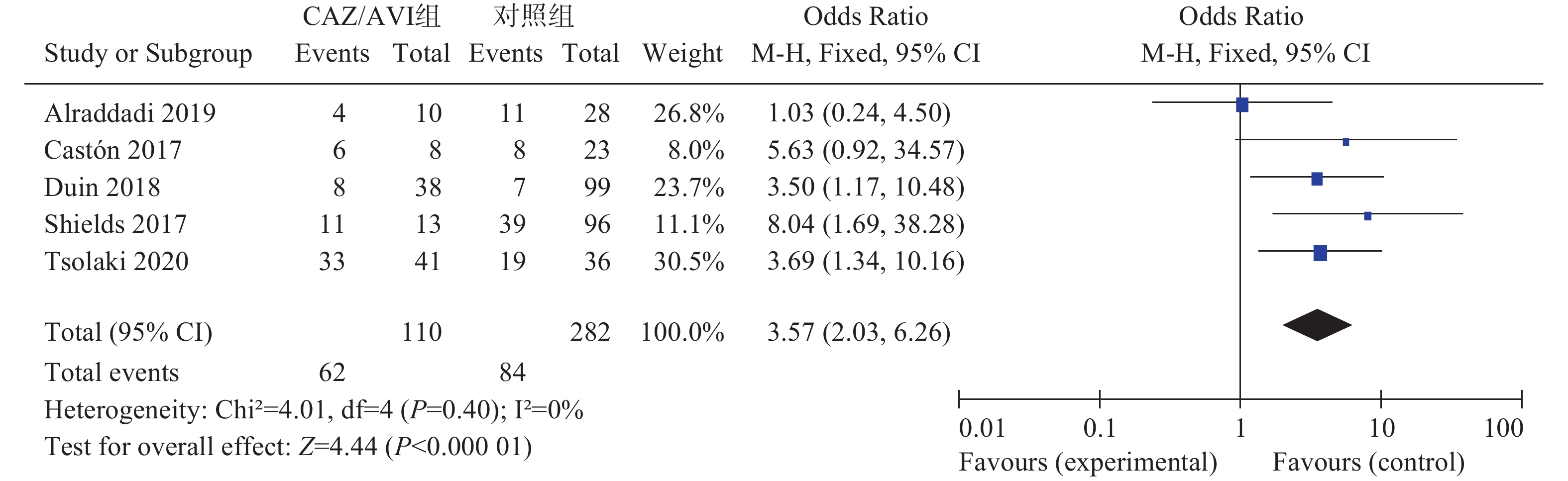
5项研究[3-7]报道了临床治愈率,各研究间无统计学异质性(I2=0%,P=0.40),选择固定效应模型分析,结果显示,CAZ/AVI组患者临床治愈率高于对照组,结果有统计学意义[OR=3.57,95% CI(2.03,6.26),P<0.00001],见图1。
-
两项研究[5-6]报道了临床缓解率,各研究间无统计学异质性(I2=0%,P=0.45),选择固定效应模型分析,结果显示两组患者临床缓解率相当,结果无统计学意义[OR=1.92,95% CI(0.93,3.97),P=0.08]。
-
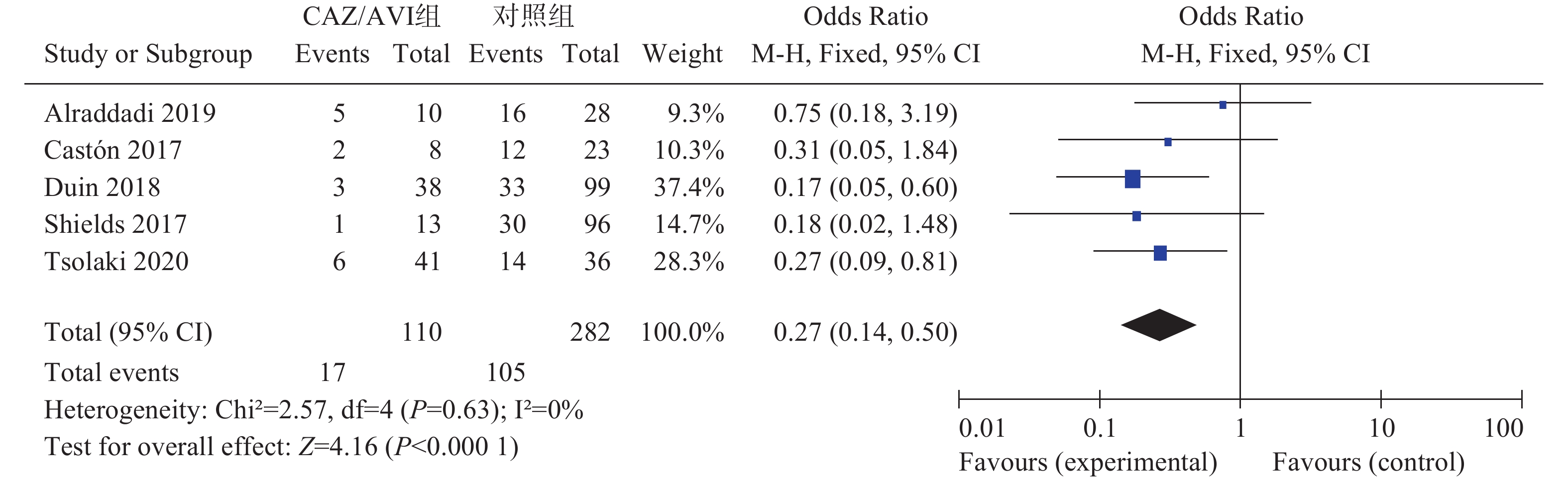
5项研究[3-7]报道了28 d/30 d全因病死率,各研究间无统计学异质性(I2=0%,P=0.63),选择固定效应模型分析,结果显示CAZ/AVI组患者28 d/30 d全因病死率低于对照组,结果有统计学意义[OR=0.27,95% CI(0.14,0.50),P<0.0001],见图2。
-
3项研究[3, 5-6]报道了感染复发率,各研究间有统计学异质性(I2=61%,P=0.08),选择随机效应模型分析,结果显示两组患者感染复发率相当,结果无统计学意义[OR=0.44,95% CI(0.11,1.85),P=0.26]。
-
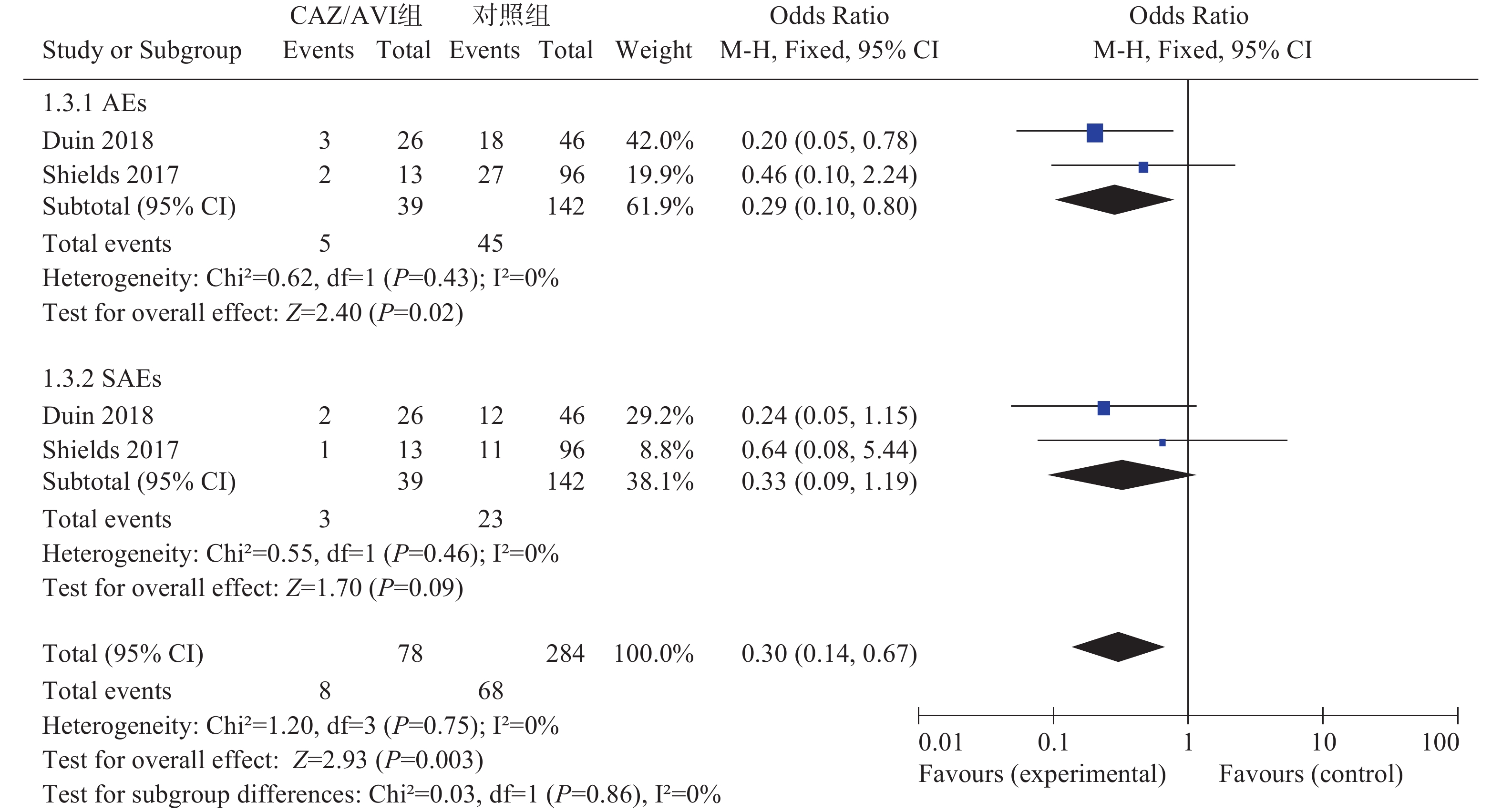
2项研究[3, 5]分别报道了不良事件(AEs)和严重不良事件(SAEs),各研究间均无统计学异质性(I2=0%,P=0.43)和(I2=0%,P=0.46),选择固定效应模型分析,结果显示,CAZ/AVI组患者AEs低于对照组,结果有统计学意义[OR=0.29,95% CI(0.10,0.80), P=0.02]。两组患者SAEs相当,结果无统计学意义[OR=0.33,95% CI(0.09,1.19), P=0.09],见图3。
2.1. 纳入研究的情况
2.2. 文献质量评价
2.3. Meta分析结果
2.3.1. 临床治愈率
2.3.2. 临床缓解率
2.3.3. 28 d/30 d全因病死率
2.3.4. 感染复发率
2.3.5. AEs和SAEs发生率
-
由于缺乏有效和安全的替代治疗方案,碳青霉烯类抗生素耐药性的上升尤其令人担忧,临床迫切需要新的抗菌药物来解决耐药性带来的治疗难题。CAZ/AVI是第三代头孢菌素和新型的非β-内酰胺类的β-内酰胺酶抑制剂阿维巴坦相结合的抗生素,将CAZ的自身疗效与AVI的抑酶作用结合,能更广泛且安全有效地对抗大多数MDR-GNB引起的感染[10]。目前CRE/CRKP对其耐药率低,但其广泛的体外活性、人们对头孢他啶比较熟悉以及耐药菌治疗药物选择严重受限等情况,可能会刺激临床对CAZ/AVI使用。本研究结果对包括392例患者的5项队列研究进行了统计分析,对CAZ/AVI治疗碳青霉烯类耐药菌感染的疗效和安全性进行了系统评价,可为临床治疗CRE/CRKP感染提供循证支持,同时可作为评价CAZ/AVI用于MDR-GNB复杂感染的系统评价的最新补充。
疗效方面,CAZ/AVI组患者临床治愈率优于对照组,CAZ/AVI组患者28 d/30 d全因病死率低于对照组,差异均有统计学意义;两组患者临床缓解率和感染复发率相当,差异无统计学意义。提示CAZ/AVI在治疗CRE/CRKP感染中疗效优于多粘菌素、替加环素、碳青霉烯类、氨基糖苷类等其他抗菌药物。Zhong等[11]通过Meta分析指出CAZ/AVI在治疗革兰阴性菌感染时,临床治愈率和细菌清除率与碳青霉烯类(多利培南、亚胺培南-西司他丁、美罗培南、厄他培南)相当,差异无统计学意义。亚组分析显示,与对照组相比,CAZ/AVI可显著提高CRE患者的临床治愈率和降低患者病死率,差异有统计学意义。同时,最新的研究[12]结果显示,在CRE感染患者治疗中,CAZ/AVI与新药美罗培南-法硼巴坦有相似的临床成功率。值得注意的是,有研究[13-14]发现,CAZ/AVI单用治疗CRE感染时常出现耐药性。
安全性方面,CAZ/AVI组患者AEs低于对照组,差异有统计学意义;两组患者SAEs相当,差异无统计学意义。研究报道[15],阿维巴坦具有较低的潜在药物相互作用,其安全性和耐受性已在CAZ/AVI研发过程中的多个临床数据中确定,包括肾功能不全和接受药物联合治疗的受试者。多项关于CAZ/AVI的Meta分析结果显示,CAZ/AVI组与对照组患者发生不良事件包括严重不良事件相当。但在一些支持CAZ/AVI作为碳青霉烯类药物替代品的研究中,有报道不良事件发生率有所增加[16-17]。同时最新研究[18]结果表明,在ESBL阳性率为25%的肠杆菌科细菌中,CAZ/AVI组患者SAEs明显高于碳青霉烯类组。本文中安全性结果仅纳入2项研究,同时鉴于CAZ/AVI上市时间较短,且主要用于严重复杂感染患者,该药的安全性还有待进一步评估。
本研究经过广泛而全面的检索,通过系统评价合并扩大了样本量,除感染复发率外其他结局指标均无异质性,结果可信度较高。但因存在以下局限性,结论仍应被谨慎对待。第一,本研究纳入统计分析的患者样本量较少(<400);第二,本研究纳入文献数量较少,且均为观察性研究,研究质量低于随机对照研究,且由于数据受限,所采用的研究数据都是未经过调整的混杂因素(如患者基础疾病、疾病的严重程度、感染源、敏感性实验折点、细菌对碳青霉烯类的MIC值等),各纳入研究对照组患者治疗方案及伴随疗法有所出入,这些都可能导致假阳性或假阴性结果;第三,本研究纳入研究的语种限制为中、英文,存在选择性偏倚。
综上所述,与多粘菌素、替加环素等常规抗CRE/CRKP感染治疗方案相比,CAZ/AVI抗生素可提高该类患者临床治愈率、有效降低28 d/30 d全因病死率,临床疗效较优。不良事件发生率显著低于对照组,但严重不良事件发生率相当,安全性略优。鉴于纳入研究数量较少、质量不高,本研究结论有待设计更优的大型临床随机对照试验来验证,以期为临床工作提供科学合理的参考。









 DownLoad:
DownLoad: