-
在高原地区,红景天作为一种常见的抗高原缺氧药,对其研究和开发具有重要意义。国内外对20多种红景天进行了化学成分的研究,证明红景天苷是有特殊生理、药理功能的有效单体[1],而红景天苷和苷元酪醇是迄今为止,研究最多的有效成分,且由于红景天中红景天苷的含量较高,常被用来评价红景天属植物药用价值的指标成分[2-6]。红景天临床应用广泛,对冠心病、高血压、心肌损伤、急性脑梗死等心脑血管系统疾病都有着良好的治疗作用。临床研究和现代药理学研究表明,大株红景天的主要化学成分为络醇、苷类等化合物,可通过扩张血管,增加血流量,使得心脏后负荷降低,心绞痛得以治疗;可使得抗缺氧耐受能力增强,耗氧量降低。红景天能够改善心肌细胞中的缺氧诱导因子-1(信使核糖核酸)的表达,从而改善因高原空气稀薄而引起的缺氧症状[7-9]。
目前测定红景天苷常用高效液相色谱法[10-13],但灵敏度较低,分析时间较长,为克服这一缺点,笔者采用LC-MS/MS的分析方法来检测红景天胶囊中红景天苷的含量。
HTML
-
API3200三重四极杆串联质谱仪(美国应用生物系统公司);LC-20A高效液相色谱仪(日本岛津公司);AE240电子天平(梅特勒-托利多公司);高速离心机(上海安亭科学仪器厂);涡旋混匀器(金坛市金城教学仪器厂)。
-
红景天苷对照品(美国SIGMA-ALDRICH公司);茶碱标准品(美国SIGMA-ALDRICH公司);红景天胶囊(西藏军区红景天研制中心,批号:190402、190908、180407)。
乙腈(分析纯,天津光复化工品研究所);灭菌注射用水(四川科伦药业股份有限公司);甲醇(色谱纯,德国MERCK公司);乙酸铵(色谱纯,天津市北联精细化学品开发有限公司)。
1.1. 仪器
1.2. 药品与试剂
-
色谱柱为Shim-pack XR-ODS柱(3.0 mm×75 mm,2.0 μm);流动相为乙腈-5 mmol/L乙酸铵溶液(90∶10,V/V);流速:0.40 ml/min;柱温:25 ℃;样品采集量:10 μl;分析时间为3 min。
-
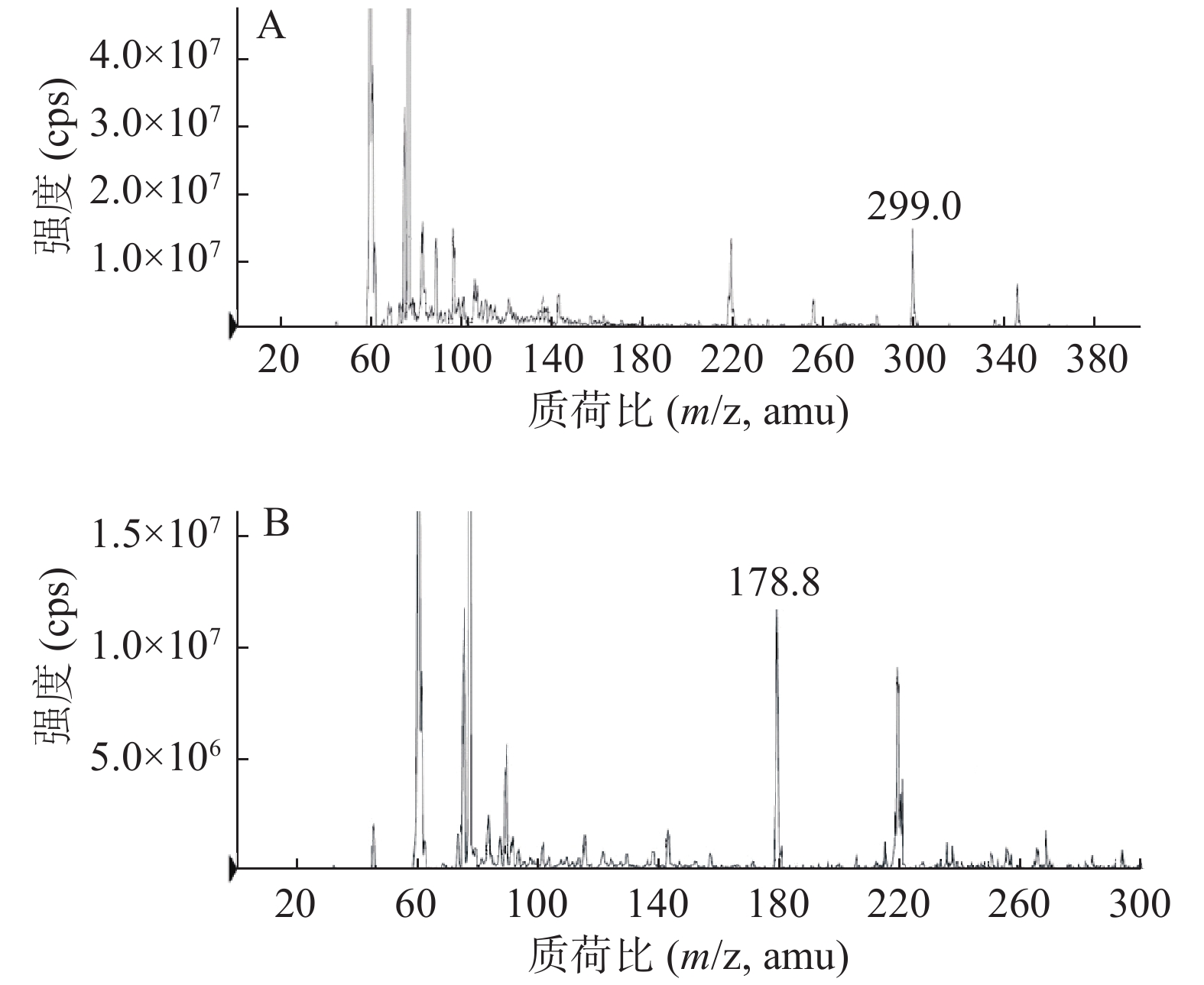
采用电喷雾离子源(ESI),负离子模式,离子源参数:雾化温度(TEM):250 ℃;喷雾电压(IS):−3700 V。多反应监测模式(MRM)参数:红景天苷和茶碱定量离子对分别为m/z 299.0→119.0,m/z 178.8→164.0,碰撞诱导解离电压(DP)均为−55 eV;碰撞能量(CE)均为−14 eV。质谱图见图1。
-
精密称取红景天苷对照品0.1 mg,置于5 ml容量瓶中,加入5 ml灭菌注射用水至刻度,摇匀,得浓度20 μg/ml红景天苷对照品储备液,4 ℃冰箱储存备用。
-
精密称取茶碱对照品0.1 mg,置于5 ml容量瓶中,加入5 ml甲醇,摇匀,配成20 μg/ml茶碱对照品储备液,4 ℃冰箱中储存备用。
-
取10粒红景天胶囊,称重,称其重量为4.8076 g。将其去除胶囊壳后,再次称重为3.6022 g。取胶囊内容物20 mg,置于5 ml的棕色瓶中,加入灭菌用水,摇匀,配制成样品溶液,将此浓度的样品作为母液,过滤,放置在4 ℃冰箱中保存用于样品浓度的测定。
-
分别精确吸取各浓度红景天苷的对照品溶液或样品溶液,置于5 ml 容量瓶中,加入茶碱对照品溶液,加甲醇稀释至刻度,混匀后,取100 μl置于0.5 ml离心管中,13 000×g高速离心10 min,离心后各吸取50 μl进行分析,进样量为10 μl。
-
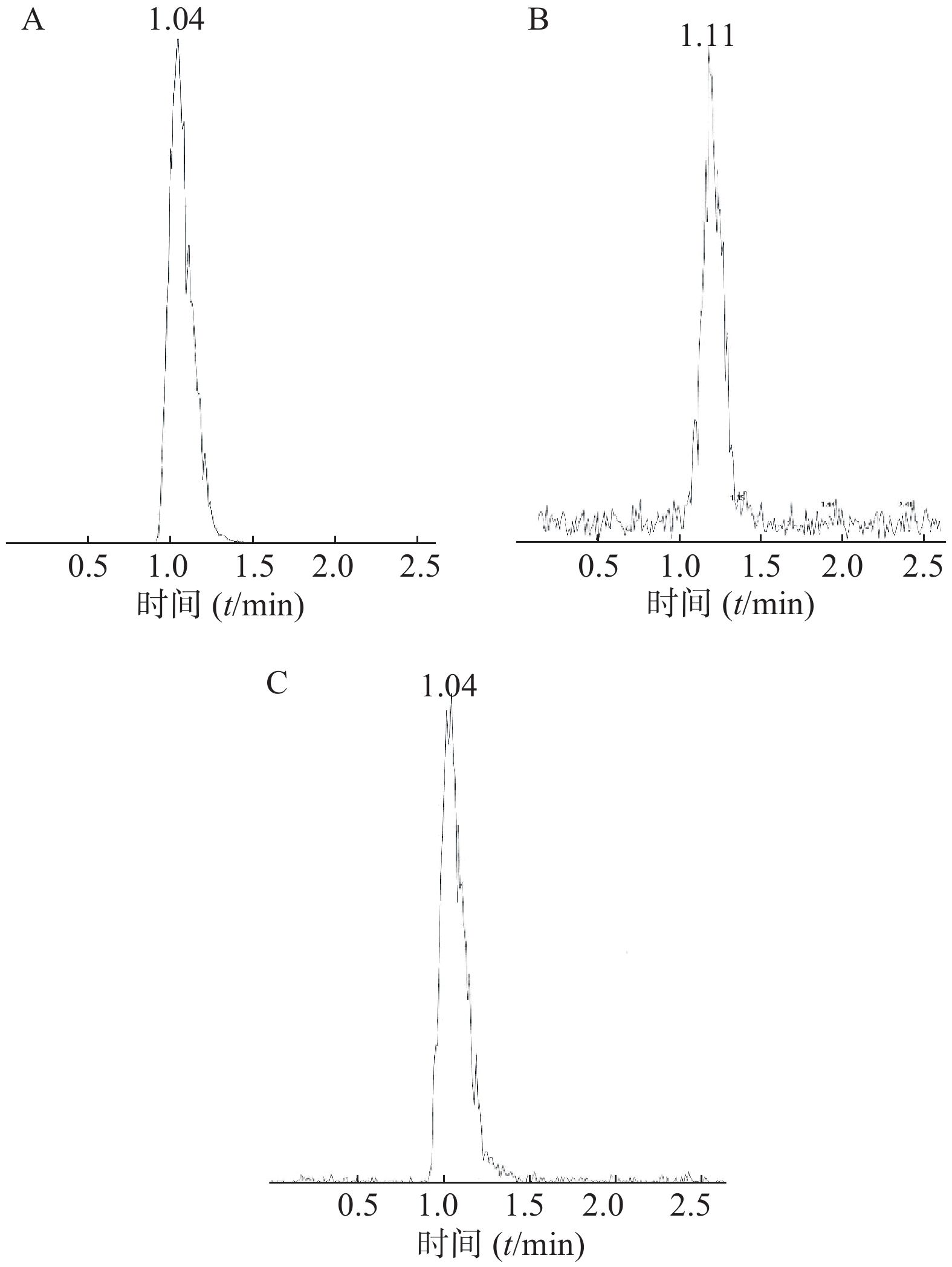
分别精密量取“2.2.1”项下制备的红景天对照品溶液,按“2.2.4”项下供试品溶液制备方法,配制成10、50、100、250、500、1000、2000ng/ml等6种不同浓度的对照品溶液,茶碱对照品浓度为50 ng/ml。依次进样,记录峰面积。以峰面积求得回归方程:Y=0.00317X−0.0291(r=0.9991)。结果表明,样品中红景天苷在10~2 000 ng/ml范围内线性关系良好。以S/N>10确定,定量下限(LOQ)10 ng/ml。对照品及供试品的色谱图如图2。
-
精密量取对照品溶液,按“2.1”项下色谱质谱条件连续进样6次,测定峰面积。红景天苷峰面积RSD为1.9%。仪器精密度良好,符合要求。
-
精密称取同一批号样品6份,按“2.2.4”项下方法制备供试品溶液,按“2.1”项下色谱质谱条件,分别进样,测定红景天苷的峰面积,RSD为2.7%(n=6),结果表明本方法重复性良好。
-
按“2.1”项下色谱质谱条件,分别精密量取在室温(10~30 ℃)下放置 0、2.5、5、7.5、10、24 h 的同一份供试品溶液进样测定,记录红景天苷的峰面积,6 次进样结果表明,供试品溶液在 24 h内基本稳定,RSD为2.8%。
-
取同一批次(批号:190402)已知含量的红景天胶囊样品9份,分别按相当于样品溶液中红景天苷含量的80%(n=3)、100%(n=3)、120%(n=3)加入“2.2.4”项下制备的供试品溶液,按“2.1”项下色谱条件进行测定。计算回收率,结果回收率在98.7%~103.2%之间,RSD为2.96%~4.21%,符合要求。
-
根据建立的红景天苷含量测定方法,3批次红景天胶囊(规格:0.3 g/粒)进行含量测定,结果见表1。
批号 红景天苷浓度(mg/粒) RSD(%) 190402 15.13 1.26 190908 15.29 2.47 180607 13.48 1.77
2.1. 色谱与质谱条件
2.1.1. 色谱条件
2.1.2. 质谱条件
2.2. 溶液的制备
2.2.1. 对照品溶液
2.2.2. 内标溶液
2.2.3. 样品溶液
2.2.4. 供试品溶液
2.3. 方法学考察
2.3.1. 线性关系考察
2.3.2. 精密度试验
2.3.3. 重复性试验
2.3.4. 稳定性试验
2.3.5. 加样回收率试验
2.3.6. 样品含量测定
-
本实验考察了流动相中不同摩尔浓度的乙酸铵对红景天苷检测的影响。当乙酸铵的浓度由2 mmol/L增大到5 mmol/L时,红景天苷色谱峰变尖锐,且灵敏度升高。综合考虑到色谱峰的峰形、响应值和分析时间,发现当乙腈∶5 mmol/L乙酸铵=90∶10(V/V)时色谱图较好。
-
本实验采用内标法,有效地消除了由于操作以及仪器本身的系统误差,提高了测量结果的精确度,特别是在药物含量较低的样品分析中,优势明显。
综上所述,所建立测定红景天胶囊中红景天苷含量的测定方法,灵敏度高、分析速度快、操作简单,为控制红景天胶囊的质量控制标准提供了方法和依据。









 DownLoad:
DownLoad: