-
伏立康唑(voriconazole)是第二代三唑类广谱抗真菌药,主要用于治疗由侵袭性曲霉菌、念珠菌等引起的侵袭性感染[1-2]。伏立康唑血药浓度受多种因素影响,其中,肝脏和肾脏疾病[3]是导致伏立康唑较大个体化差异的重要因素,伏立康唑的临床疗效及不良反应与药物浓度有明显相关性[4]。目前用于伏立康唑的血药浓度监测的方法主要有:酶联免疫法、高效液相色谱法[5],超高效液相色谱-串联质谱法[6],其中,酶联免疫法存在假阳性,高效液相色谱法检测时间较长。本研究采用的超高效液相色谱-串联质谱检测方法灵敏度高,简便快速,适用于伏立康唑血药浓度监测。由于伏立康唑大部分在肝脏代谢,肝脏疾病患者的血药浓度具有可变性和不可预知性,尤其是肝功能不全的患者,治疗药物监测对其尤为重要。然而,国内针对肝功能不全患者的伏立康唑药物浓度监测鲜有报道,笔者应用自建方法对肝功能不全患者进行伏立康唑治疗药物血药浓度监测,根据患者临床症状和生化指标及时调整用药方案,避免了药物不良反应的发生,具有一定的临床意义。
HTML
-
LC-30AT 高效液相色谱仪(日本岛津公司);AB Qtrap 5500型质谱仪,配有电喷雾电离源 (美国AB SCIEX公司)。AL 204型分析天平[梅特勒-托利多仪器(上海)有限公司];Simplicity纯水仪(美国Millipore公司);H1850R 低温高速离心机(湘仪离心机仪器有限公司);XH-B 型旋涡混合器(湖北姜堰市康健医疗器具有限公司)。伏立康唑对照品(中国食品药品检定研究院,批号:100862-201402,纯度99.8%);氟康唑对照品(中国食品药品检定研究院,批号:100314-201605,纯度99.8%);甲醇、乙腈为色谱纯,其他试剂均为分析纯。
-
色谱柱:InertSustain C18 HP(3.0 mm×100 mm,3 μm);流动相:水 -乙腈(15∶85);流速: 0.3 ml/min,柱温:35 ℃;进样量:1 μl。
-
电喷雾离子源(ESI),正离子多反应监测(MRM)模式监测。喷雾电压(IS)5 550 V;离子化温度(TEM)550 ℃;雾化气(GS1)55 psi;辅助气(GS2)60 psi;气帘气(CUR))20 psi;碰撞气(CAD)10 psi;去簇电压(DP)80 V。伏立康唑和内标氟康唑的定量分析离子分别为m/z:350.9/282.2和m/z:307.0/238.0;碰撞能量分别为25 V和22 V。
-
精密称取伏立康唑适量,置10 ml容量瓶中,用甲醇溶解并稀释至刻度,得1 mg/ml伏立康唑储备液,置2~8 ℃冰箱保存,备用。
-
精密称取氟康唑适量,置于10 ml容量瓶中,用甲醇溶解并稀释至刻度,得1 mg/ml的氟康唑储备液,置2~8 ℃冰箱保存,备用。临用前用甲醇稀释成500 ng/ml的内标溶液。
-
取样本血浆50 μl,加入500 ng/ml内标液50 μl,加入甲醇250 μl,在微型混合器上涡流振荡1 min混匀,13000 r/min低温4 ℃离心5 min,取上清液1 μl进样分析。
-
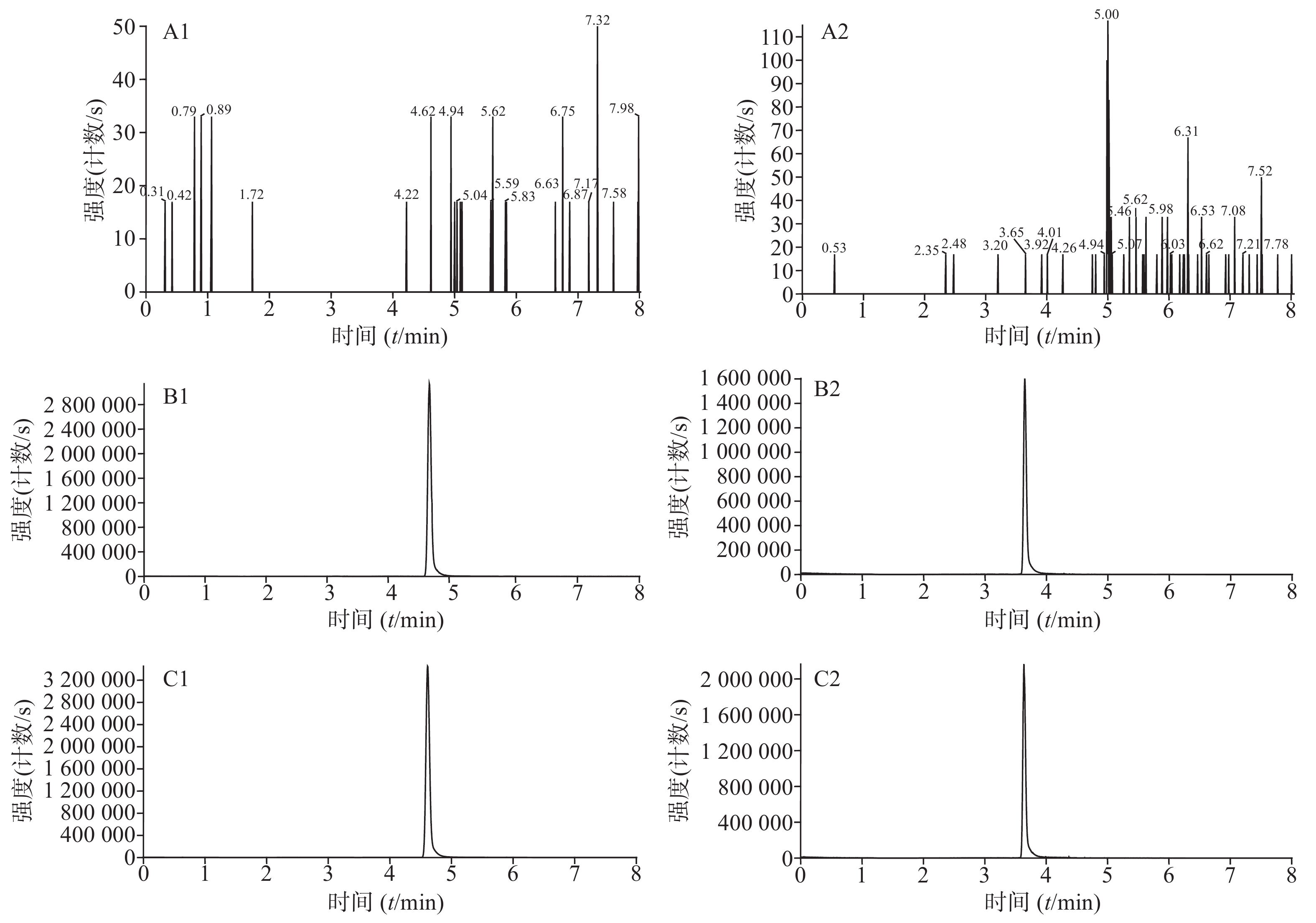
分别取人空白血浆、人空白血浆加伏立康唑对照品和内标溶液、临床使用伏立康唑的患者血浆样品加内标溶液,按照“2.4”项下方法处理,按“2.1”和“2.2”项下条件进行检测。伏立康唑和内标的保留时间分别为4.61、3.64 min,峰形良好,血浆中其他成分对伏立康唑和内标的测定无干扰,方法的专属性好。结果见图1。
-
取不同来源的健康人血浆,精密加入不同浓度的对照品溶液,配制成浓度为0.1、0.5、1、2、5、10、20 μg/ml的标准曲线工作液。以伏立康唑浓度为横坐标,伏立康唑峰与内标峰面积的比值为纵坐标,采用最小二乘法进行加权回归计算,回归方程为:Y=0.732 36 X−0.013 55(r=0.999 5)。结果表明,人空白血浆中伏立康唑在0.1~20 μg/ml内线性关系良好。本方法伏立康唑定量下限(S/N>10)为0.1 μg/ml。
-
以人空白血浆为基质,制备浓度分别为0.3、8、15 μg/ml的低、中、高3 个水平的质控样品各6 份,连续检测3 d,按“2.4”项下方法处理后分析测定,计算其日内、日间精密度,结果见表1。
浓度(μg/ml) 日内 日间 提取
回收率
(%)基质
效应
(%)$\bar{x}\pm s$ 精密度(%) $\bar{x}\pm s$ 精密度(%) 0.3 0.27±0.01 3.47 0.28±0.01 3.43 93.57 88.85 8 7.75±0.40 5.13 7.66±0.08 1.09 91.05 90.37 15 14.32±0.29 2.04 14.69±0.41 2.76 92.46 93.61 -
取6个不同来源的健康人空白血浆作为基质,制备浓度分别为0.3、8、15 μg/ml的低、中、高3 个水平的质控样品,按“2.4”项下方法处理并测定,得到伏立康唑峰面积A1;以6个不同来源的人空白血浆甲醇沉淀提取液配制浓度分别为0.3、8、15 μg/ml的低、中、高3 个水平的样品各6 份,得到伏立康唑峰面积A2;以流动相代替空白血浆,同样方法配制备0.3、8、15 μg/ml的低、中、高3 个水平的样品各6 份,得到伏立康唑峰面积A3;以A1/A2的值计算提取回收率,A2/A3的值计算基质效应,结果见表1。
-
以人空白血浆为基质,制备浓度分别为0.3、8、15 μg/ml的质控样品,考察样品室温放置稳定性(12 h)、冻融稳定性(反复冻融3次)、冷冻稳定性(−20 ℃放置30 d),结果见表2。
浓度(μg/ml) 室温放置12 h 反复冻融3次 −20 ℃放置30 d $\bar{x}\pm s$ RSD(%) $\bar{x}\pm s$ RSD(%) $\bar{x}\pm s$ RSD(%) 0.3 0.30±0.01 2.83 0.28±0.01 5.32 0.31±0.02 5.31 8 7.98±0.21 2.62 8.15±0.27 3.31 7.87±0.30 3.78 15 14.56±0.63 4.35 14.51±0.56 3.89 15.08±1.02 6.79 -
对本院10名真菌感染使用伏立康唑的肝功能不全患者进行治疗药物监测,稳态谷浓度最大值为18.7 μg/ml,最小值为0.97 μg/ml。在 Pascual 等[7] 的研究中,设定伏立康唑的治疗药物浓度范围为 1.0~5.5 μg/ml。其中一名患者肝硬化、胆石症伴梗阻性黄疸,行经内镜逆行性胰胆管造影术(ERCP)后胆红素、C-反应蛋白(CRP)、降钙素原(PCT)进行性升高,存在胆管感染,且患者反复腹泻,合并肠道真菌感染,予加用伏立康唑抗真菌治疗。用药前肌酐值81 μmol/L,碱性磷酸酶259 U/L,谷氨酰转肽酶69 U/L,谷草转氨酶116.3 U/L,伏立康唑胶囊口服给药,负荷剂量400 mg,q12 h,维持剂量为200 mg,q12 h,达到稳态谷浓度后监测浓度值为18.7 μg/ml,复查生化,肌酐值151 μmol/L,碱性磷酸酶324 U/L,谷氨酰转肽酶154 U/L,谷草转氨酶154 U/L,胆红素仍持续性升高,伴肾功能不全,为防止发生严重肝损害和不良反应,予停药处理。还有一名患者肝炎后肝硬化、肝功能衰竭伴黄疸,粪便培养出黑霉菌,予伏立康唑抗真菌治疗,用药前肌酐值49 μmol/L,碱性磷酸酶198 U/L,谷氨酸转氨酶99 U/L,谷草转氨酶211 U/L。伏立康唑片剂口服给药,负荷剂量400 mg,q12 h,维持剂量为200 mg,q12 h,达到稳态谷浓度后监测浓度值为10.7 μg/ml,已超出伏立康唑治疗窗浓度高限,患者诉视物变黄,初步考虑为药物引起的不良反应,临床医生调整剂量为 100 mg,q12 h,达到稳态浓度后复测伏立康唑的血药浓度为5.1 μg/ml,视物变黄较前缓解,无视物模糊,黄疸下降,凝血酶原活动度(PTA)改善,感染症状有所好转。
2.1. 色谱条件
2.2. 质谱条件
2.3. 溶液的制备
2.3.1. 对照品溶液
2.3.2. 内标溶液
2.4. 样品处理方法
2.5. 专属性考察
2.6. 线性关系考察
2.7. 精密度试验
2.8. 提取回收率和基质效应
2.9. 稳定性试验
2.10. 临床样本测定
-
在色谱法测定伏立康唑血药浓度的文献中,前人多采用乙酸乙酯-环己烷或者三氯甲烷萃取剂提取[8-9],前处理的过程操作比较复杂,时间长。本研究使用甲醇蛋白沉淀方法,能够较好地去除血浆样本中内源性物质的干扰,降低基质效应对检测的影响,前处理方法简单快速,缩短了检测时间。所选内标氟康唑稳定性好,保留时间适宜且峰形好,定量准确,同时,液相方法使用乙腈-水等度洗脱,将分析时间缩短至6 min以内,分析效率显著提高。综上所述,本研究建立的伏立康唑血药浓度的测定方法具有较好的专属性,操作简便,回收率好、灵敏度高,可满足伏立康唑日常血药浓度的测定和药动学研究的需要,为合理使用伏立康唑提供依据。
伏立康唑谷浓度低于治疗窗最低浓度时临床治疗效果不好,当谷浓度高于治疗窗最大浓度时,中枢系统[10]和肝功能[11]的不良反应发生率会明显升高。其中,视觉障碍最为常见,与用药剂量较大或血药浓度较高有关,一般用药剂量减少或停药后可完全恢复。患者个体化差异和药物间相互作用等因素会导致血药浓度偏高或偏低,影响伏立康唑的治疗效果。此外,患者肝功能不全会影响伏立康唑的代谢,导致代谢时间减慢,半衰期延长,药物不良反应的发生率增加,从而使得肝损害进一步加重。
本例中两名患者肝功能损害较严重,伏立康唑用药需更加谨慎。伏立康唑在脑脊液和脑组织液中可达到较高浓度,有研究表明伏立康唑药物浓度与视觉副作用的发生率呈正相关,临床药师通过监测伏立康唑血药浓度,及时调整用药,结合患者实际病情制订个体化给药方案,提高治疗效果,避免了药物不良反应的发生,为临床肝功能不全患者合理用药提供依据。








 DownLoad:
DownLoad: