-
阿尔茨海默症(Alzheimer's disease,AD)是常见的神经系统变性疾病之一,是一种持续性神经功能障碍,也是痴呆最常见的病因,其发生可导致进行性记忆减退、认知障碍、人格改变等症状。65岁以上患病率约5%,85岁以上患病率高于20%,是老年人死亡的主要原因之一[1-3]。AD的病理特征主要是老年斑(senile plaques,SP)、神经纤维缠结(neurofibrillary tangles,NFTs)和广泛神经元缺失。tau蛋白是一种微管相关蛋白,过度磷酸化tau蛋白是造成神经纤维缠结的主要原因,且AD患者病情严重程度与tau蛋白具有明显相关性。因此,tau蛋白显像剂的研究逐渐受到关注[4-5]。
近年来,研究者们研发了多种tau蛋白的PET显像剂,如“THK系列”(包括18F-THK5105、18F-THK523、18F-THK5117、18F-THK5351)[6-8],“RO系列”(包括18F-RO6958548、11C-RO6931643、11C-RO6924963)[9-11],“T系列”(包括18F-T807、18F-T808)[12]以及11C-PBB3[13]等。其中“T系列”18F-T807和18F-T808是由Simens公司开发的tau蛋白的分子探针。
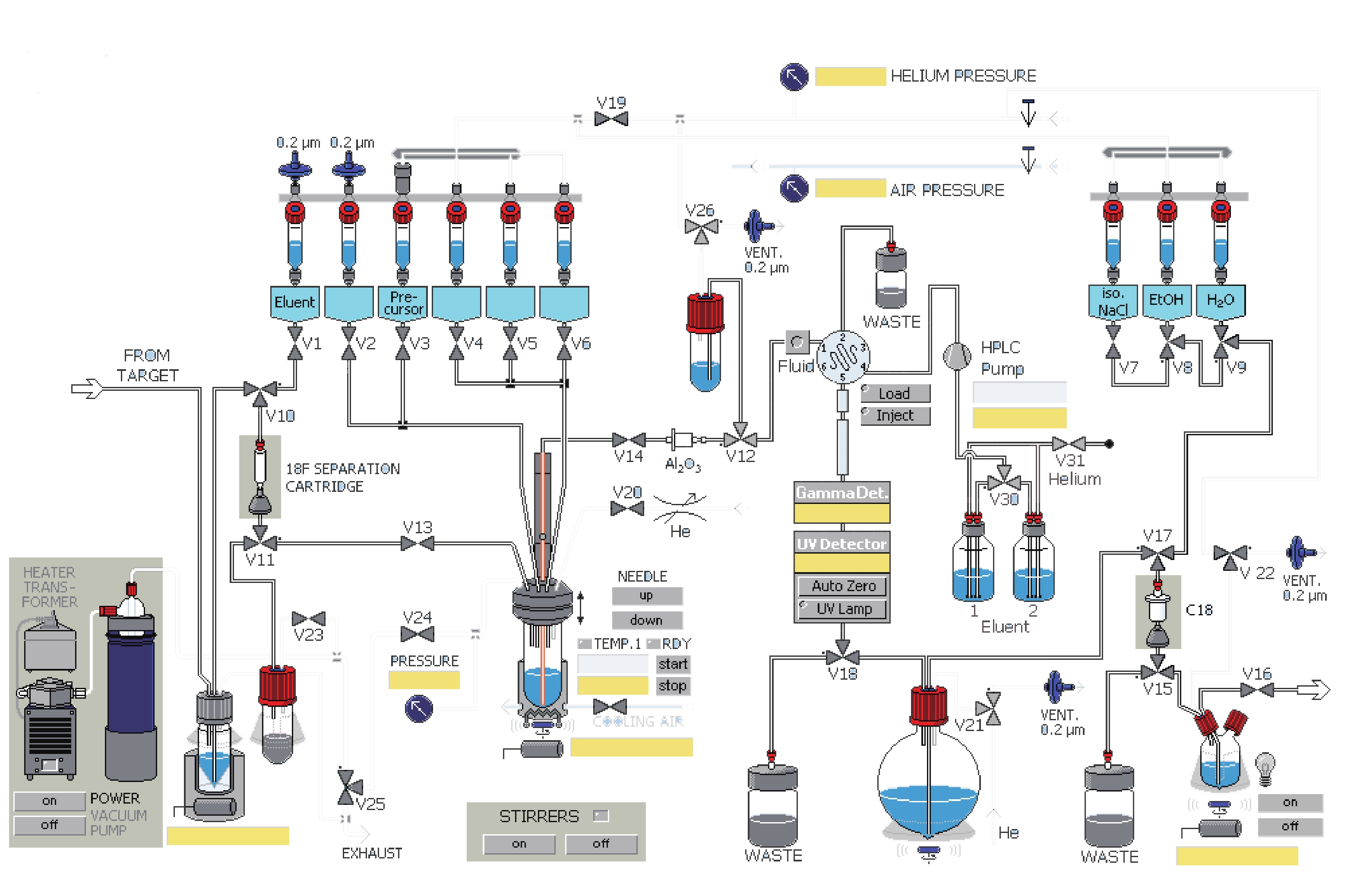
课题组在参考相关文献的基础上[14-15],使用GE公司的TRACERlab FXFN氟多功能合成模块(图1),以18F-T807前体(BOC保护)NPPI-95(1)为原料,使用改进一锅法自动合成了18F-T807(2),提高了产品产率,并开展初步的正常大鼠生物分布实验,探索其分布特征。
-
18F-T807前体(BOC保护)NPPI-95(江苏华益公司);强阴离子交换固相萃取柱(QMA柱)、K222、碳酸钾水溶液、乙腈、(德国ABX公司);乙醇(国药化学试剂);盐酸(国药分析质检中心);DMSO(北京百灵威);Ultimate C18柱(美国Waters: 4.6 mm×250 mm,5 μm);富18O水(日本大阳日酸株式会社);0.22 μm MILLEX-GS液体滤膜、0.2 μm Millex-25空气滤膜(德国默克);0.7 mm×40 mm针头(西班牙BD Microlance);Wistar大鼠(北部战区总医院实验动物科)。溶剂乙醇为色谱纯,其余均为分析纯。
TRACERlab FXFN(美国GE)配备半制备VP 250×16高效液相色谱(德国MN)和紫外检测器及放射性检测器;正电子示踪剂质量控制薄层扫描仪(美国Bioscan),配塑料闪烁体晶体探测器;分析用HPLC(北京优联);GC-7900气相色谱(北京天美);CRC 25R活度计(美国Capintec),合成条件满足药品生产质量管理规范(GMP)的要求。
-
溶剂瓶 溶剂 1号瓶(V1) 1.5mg K2CO3溶于0.5 ml水 2号瓶(V2) 1.5 mg K222溶于1ml乙腈 3号瓶(V3) 1 mg前体溶于1.2 ml DMSO溶剂 5号瓶(V5) 1.5 ml HPLC流动相 6号瓶(V6) 1.5 ml HPLC流动相 圆底烧瓶 2 ml 84%NaHCO3水溶液和30 ml水 7号瓶(V7) 9 ml 0.9%生理盐水 8号瓶(V8) 1 ml 乙醇 9号瓶(V9) 10 ml 水 18F-T807自动化合成主要有以下几步:①18F-离子的柱分离纯化及蒸馏干燥。②T807前体的18F-离子亲核取代反应。③18F-T807的HPLC分离纯化。④18F-T807 C18柱溶剂转换与再纯化。
自动合成的具体步骤如下:
(1)共2.5 ml含18F-离子的18O水由MINItrace加速器经由18O(p, n)18F反应制备,轰击束流45μA,轰击时间40 min,18F-离子混合液由氦气作为载气经过TARGET管线传输到TRACERlab FXFN合成模块的锥形瓶内。
(2)V10、V11号阀门开启,18F-离子及18O水混合液中的18F-离子在真空泵抽取下被QMA柱(由1 ml乙醇,2 ml水活化)捕获滞留,18O水回收进入18O水回收瓶。
(3)V1、V13、V24号阀门开启,V1号瓶内的K2CO3溶液流经V1、V10、QMA柱、V11、V13,将18F-离子交换抽入反应瓶。
(4)关V1、V13号阀门,开启V2号阀门将V2号瓶内穴醚K222乙腈溶剂抽入反应管,18F-离子进入穴醚形成复合物。
(5)关V2号阀门,开启V20号阀门混合液在氦气吹拂下于85 ℃共沸蒸馏8 min,然后加热到110 ℃,在氦气吹拂下共沸蒸馏4 min除水。
(6)开启V3、V19号阀门,在氦气推动下V3号瓶内的前体流入反应管,V3、V19、V24号阀门关闭,反应管加热到140 ℃,反应10 min。
(7)反应瓶降温到50 ℃,开V24、V25号阀门恢复大气压。
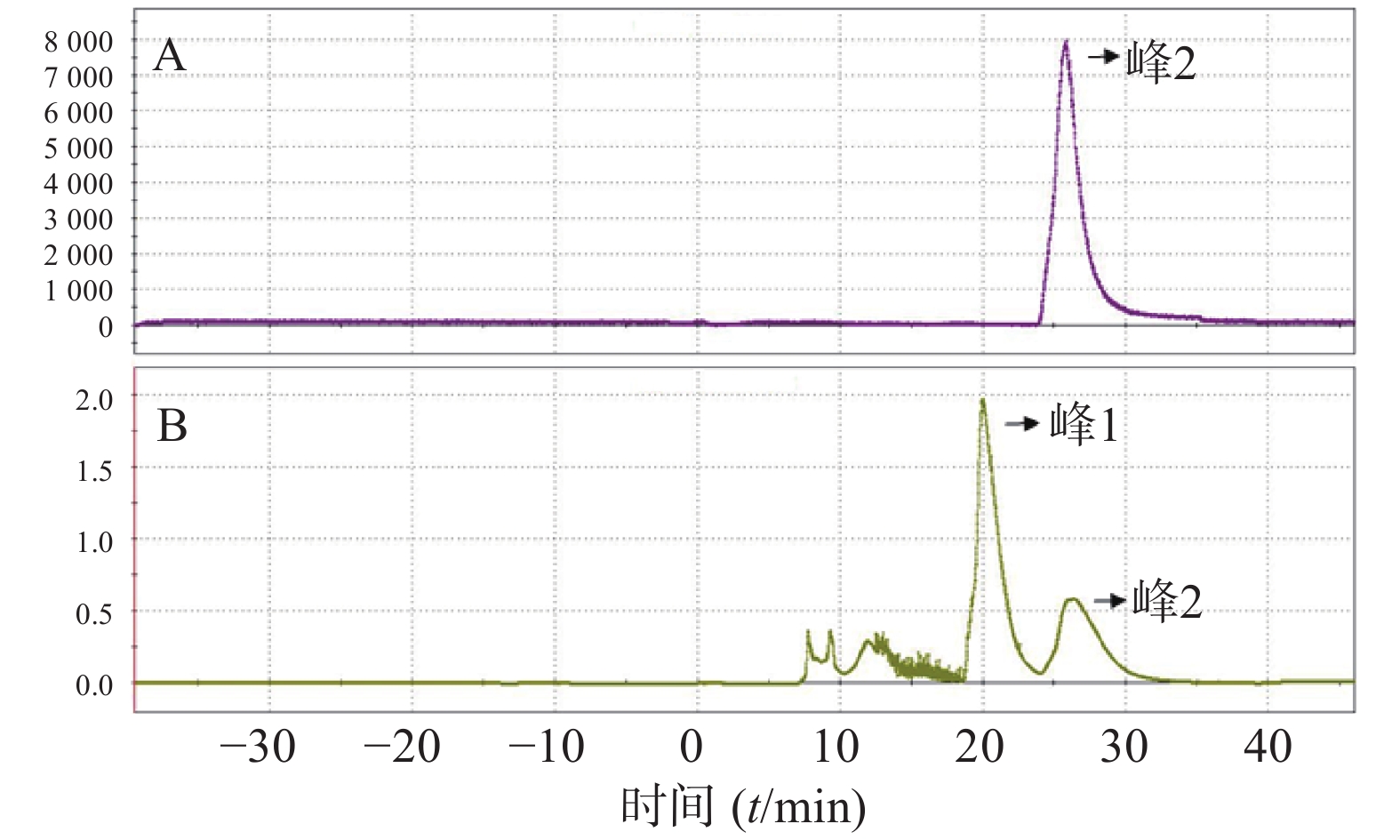
(8)反应后混合液经由V5、V6号瓶内的共3 ml HPLC流动相(25%乙醇水溶液,调整pH至2.0)冲洗到V26号阀门下的中转瓶内,然后打开V26、V12号阀门,在氦气压力下经由Fluid进入HPLC进样环,在Fluid控制下进样环旋转,产物进入HPLC半制备柱,Eluent1号瓶内流动相以5 ml/min的流速通过柱子分离。流动相以紫外(UV,λ=254 nm)和放射计数器监测。图3是18F-T807的HPLC及UV图。
(9)18F-T807溶液通过V18号阀门进入圆底瓶,圆底瓶内装有2 ml 84% NaHCO3水溶液和30 ml无菌注射用水。然后经V21、V15、V17号阀门,产物溶液通过V15、V17号阀门间的C18柱(以5 ml乙醇和10 ml水活化),产物会被捕获滞留在柱子上,然后打开V9阀门,用V9号瓶内10 ml水冲洗柱子到废液瓶(WASTE)内,然后C18柱经由V8号瓶内的1 ml乙醇冲洗进入V15阀门下的产品瓶,再经由V7号瓶内装有9 ml生理盐水再次冲洗。
(10)手动打开V22和V16号阀门,18F-T807在氦气压力下经过0.22μm液体滤膜过滤进入分装热室的收集瓶。
-
对3批连续生产的产物进行了质量控制。质控项目包括澄明度、pH、核素半衰期、核素纯度、放化纯度、K222和残留溶剂、细菌内毒素、无菌测试,测试结果均符合标准要求。
-
选择健康雄性Wistar大鼠30只,分为6组,每组5只,实验前6 h禁食禁水,每只通过尾静脉注入0.2 ml(约7.4 MBq)的18F-T807后,分别在5、15、30、60、90、120 min断头处死,取出脑、心、肝、肺、肾、肌肉、骨和血,去污、称重、计数,数据经衰减校正后计算放射性摄取率(每克组织的放射性摄取剂量占注射剂量的百分比)。
-
18F-T807有多种合成方法,本文在参考相关文献报道基础上,优化反应条件,改变前体用量为1 mg,同时使用HPLC分离条件为25%乙醇水溶液, pH调整至2.0,在线脱BOC保护。C18柱溶剂转换与再纯化,应用经改进的合成方法使合成产率由(20.5±6.1)%提高到(25.7±5.8)%,总反应时间为70 min。
连续3批产品,其质量控制结果如下:肉眼观察溶液无色透明,6 h后pH值为7,半衰期满足要求,不包含长半衰期核素(t1/2>5天),核素纯度大于99.5%,HPLC和TLC分析结果,即化学纯度和放化纯度合格,流动相是50%甲醇/水(HCl调节pH至2,),流速1.3 ml/min,紫外检测波长为254 nm,TLC条件为NH3H2O-甲醇-CH2Cl2 (1∶5∶94),气相色谱结果显示残留的丙酮、乙腈、DMSO等溶剂均在检测线下,细菌内毒素实验(鲎试剂法)合格,无菌检查合格。各项结果表明产品符合人体使用标准。
正常大鼠18F-T807在体内的生物分布如表2所示,可见大部分器官在给药5 min后摄取率最高,其中肾、肝、血的摄取率较高,超过5.56%ID/g(%ID/g为放射性摄取率,即各器官的每克放射性摄取值),在肌肉、骨骼摄取率相对较低,因此推断18F-T807主要是经过肝肾排出体外。18F-T807的脑、心、肺摄取率最低,120 min已降低至本底水平(1.08% ID/g),各器官的放射性摄取率随时间的推移逐渐降低,但清除较慢,在120 min 时大部分器官仍有较高的摄取率。
器官 放射性摄取率(% ID/g) 5 min 15 min 30 min 60 min 90 min 120 min 脑 2.25±0.18 2.03±0.86 1.81±0.54 1.59±0.62 1.20±0.57 1.11±0.38 心 2.05±0.58 1.99±0.66 1.78±0.31 1.55±0.25 1.19±0.74 1.08±0.36 肝 5.79±2.58 5.95±1.17 5.48±0.66 5.29±0.71 4.83±0.84 4.27±0.86 肺 2.12±0.91 2.01±0.56 1.91±0.19 1.57±0.73 1.21±0.52 1.09±0.23 肾 7.36±4.01 5.11±1.21 3.89±1.99 3.63±1.82 3.17±1.68 2.99±0.98 肌肉 2.34±0.86 2.57±1.18 2.44±0.95 2.19±1.36 2.04±1.03 1.51±0.89 骨 2.58±0.91 2.67±0.75 2.02±0.68 1.99±0.82 1.52±0.46 1.27±0.55 血 5.56±0.35 5.41±0.56 4.73±0.74 4.57±1.31 4.22±0.37 4.01±0.45 -
在TRACERlab FXFN合成器上使用优化条件的一锅法自动合成了18F-T807,提高了产品产率。合成后进行的各种质量控制检测均显示产品符合质控标准。初步的正常大鼠生物分布实验,显示了其不同时间放射性摄取率的分布情况,为应用该产品开展人体显像提供了重要基础。
Synthesis method optimization and biodistribution study of 18F-T807 on TRACERlab FXFN synthesizer
doi: 10.12206/j.issn.1006-0111.202009023
- Received Date: 2020-09-08
- Rev Recd Date: 2021-09-09
- Available Online: 2021-12-27
- Publish Date: 2021-11-25
-
Key words:
- T807 /
- tau protein /
- radiopharmaceutical /
- PET
Abstract:
| Citation: | ZUO Feng, HUO Hua, WANG Zhiguo, ZHANG Guoxu, SHI Qingxue, ZHANG Zongpeng. Synthesis method optimization and biodistribution study of 18F-T807 on TRACERlab FXFN synthesizer[J]. Journal of Pharmaceutical Practice and Service, 2021, 39(6): 525-528. doi: 10.12206/j.issn.1006-0111.202009023 |









 DownLoad:
DownLoad: