-
根据WHO的数据,每年约有399 000人死于与丙型肝炎病毒(hepatitis C virus, HCV)感染有关的肝病。目前,尚没有疫苗可用于预防HCV感染。HCV是黄病毒科中一种有包膜的正链单链RNA病毒。HCV基因组RNA编码为一种多聚蛋白,这种多聚蛋白可以被宿主和病毒的蛋白酶切割转化为成熟蛋白,其中包括:核心蛋白、糖蛋白E1和E2(组成结构蛋白)、离子通道p7和非结构蛋白,包括NS2、NS3、NS4A、NS4B、NS5A和NS5B等。非结构病毒蛋白NS3/4A、NS4B、NS5A和NS5B组装并与宿主蛋白相互作用,形成病毒复制复合物。非结构蛋白之一的NS5B是一种HCV复制酶,它也是一种RNA依赖的RNA聚合酶(RdRp),可以复制病毒RNA的基因组[1]。因此,NS已经成为药物开发的主要目标[2]。这类以NS蛋白为靶点的抗丙肝药物,可以直接杀死HCV,也称为直接抗病毒药,例如,蛋白酶抑制剂特拉匹韦(telaprevir)或西咪匹韦(simeprevir)和RNA聚合酶抑制剂索磷布韦(sofosbuvir)对大多数常见HCV基因型的有效率高达90%,从而提高了持续的病毒学应答率。但是,这些直接抗病毒药治疗可能会导致高昂的治疗费用以及一些其他问题:如耐药性、药物相互作用,以及疲劳、头痛、恶心、失眠、甚至贫血等不良反应。因此,针对HCV生命周期不同阶段的新型药物可能为抗HCV耐药性发展和感染复发等提供有前途的方法。
从天然产物中寻找和发现新的活性化合物一直是新药研究的主要方向之一。近些年来,具有抗HCV作用的天然化合物越来越受到重视。本文对近年来报道的具有较好活性的天然抗HCV活性化合物做一综述,为天然产物抗HCV研究提供一些思路。
HTML
-
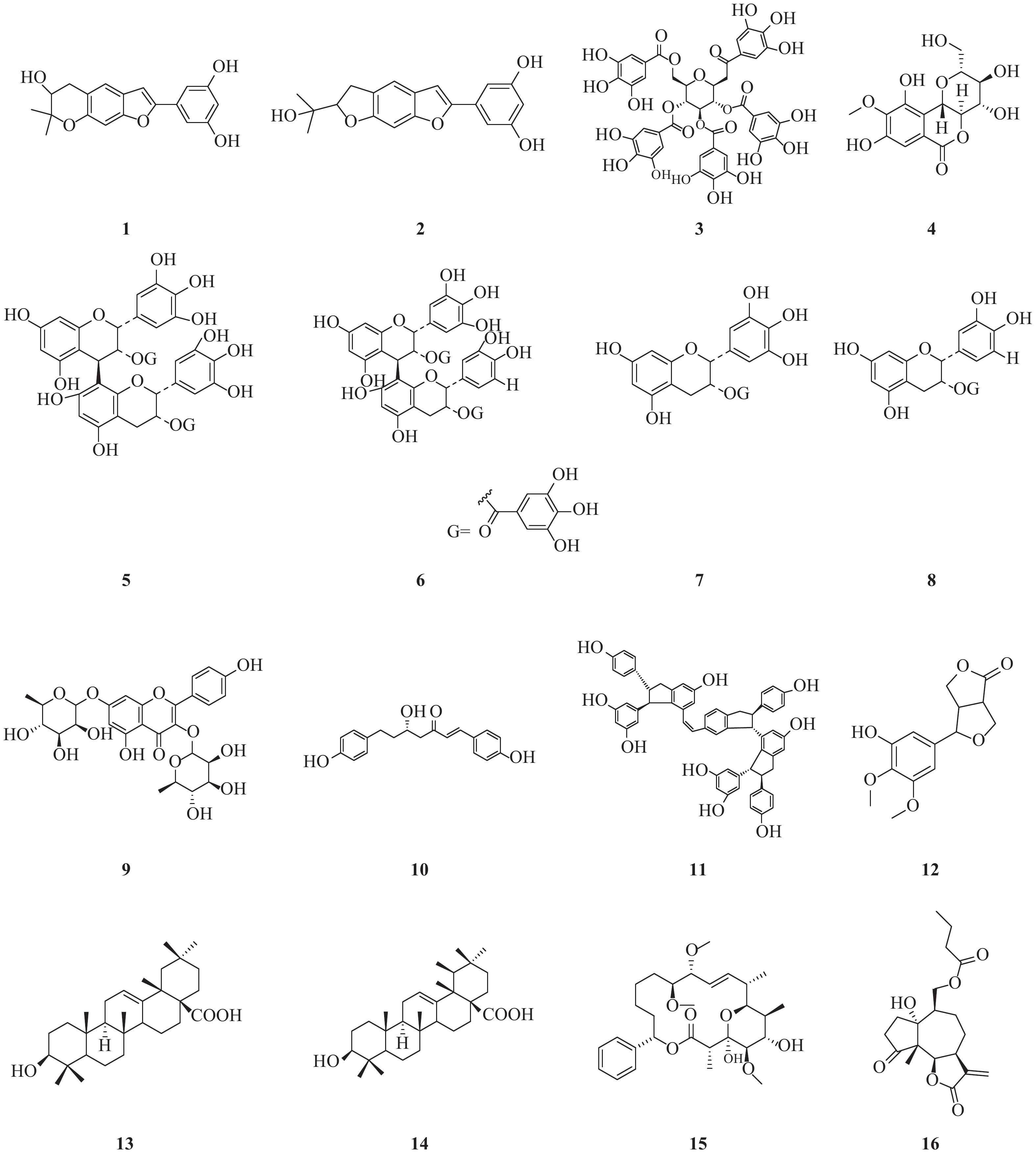
NS3作为HCV的一种重要的非结构蛋白,在HCV复制过程中起着重要的作用。NS3是一种丝氨酸蛋白水解酶,也是病毒解旋酶,可以解螺旋RNA×RNA和RNA×DNA二倍体,对病毒复制是非常重要的酶。因此,以NS3为靶标寻找抗HCV化合物也是抗HCV药物研究重要方向之一(图1)。Lee等[3]报道从中药桑白皮的乙醇提取物中分离鉴定了一类2-芳基苯并呋喃类化合物。其中moracin P(1)和moracin O(2)在这类化合物中显示出对HCV较强的抑制活性,IC50分别为35.6和80.8 μmol/L;对NS3的抑制活性IC50分别为42.9和27.0 μmol/L。
Zuo等报道了从黑蕊虎耳草(Saxifraga melanocentra Franch)中分离得到了一类具有HCV的NS3丝氨酸蛋白水解酶抑制活性的多酚类化合物[4]。其中化合物1,2,3,4,6-五-O-没食子酰-β-D-葡萄糖苷(3)、1,4-二-O-没食子酰-3,6-(R)-六羟基二苯甲酰基-β-D-吡喃葡萄糖(石榴皮鞣质)、1,2,4,6-四-O-没食子酰-β-D-葡萄糖苷、1,2,4-三-O-没食子酰-3,6-六羟基二苯甲酰基葡萄糖(石榴叶鞣质)和1,3,6-四-O-没食子酰-β-D-葡萄糖苷,对NS3丝氨酸蛋白水解酶抑制活性IC50分别为0.68、0.76、0.81、0.85和1.01 μmol/L。没食子酸、没食子酸甲酯、虎耳草素(4)、山奈酚、山奈酚-3-O-β-D-葡萄糖苷和2′-O-没食子酰芦丁,对NS3丝氨酸蛋白水解酶抑制作用IC50分别为1.76、1.36、1.71、1.61、1.03和4.86 μmol/L。而槲皮素和芦丁对NS3的作用相对较弱,IC50为33.11和77.05 μmol/L。从构效关系可以发现,较多的没食子酰结构有利于提高抑制NS3活性。
Zuo等[5]报道了从狭叶红景天Rhodiola kirilowii (Regel) Maxim中分离得到了4个(-)-表儿茶素衍生物:红景天素(5)、3,3′-二没食子酰基原花青素(6)、(-)-表没食子儿茶素-3-O-没食子酸酯(7, EGCG)和(-)-表儿茶素-3-O-没食子酸酯 (8, ECG),它们均显示出较好的抗HCV NS3丝氨酸蛋白水解酶活性,其IC50分别为0.77、0.91、8.51和18.55 μmol/L。从这两个研究结果可以发现,没食子酚或者其他具有较多酚羟基结构的天然多酚类化合物具有较好的NS3丝氨酸蛋白水解酶抑制活性。
从中药桑寄生醇提物的乙酸乙酯部位分离出10种化合物,其中山奈酚-3,7-双鼠李糖苷(19.4 μmol/L, 9)和(3S)-3-羟基-1,7-双(4-羟基-苯基)-6E-庚烯-5-酮(28.7 μmol/L, 10)显示出最强的抗HCV NS3蛋白酶抑制活性,其他分离得到的黄酮类化合物和二芳基庚类化合物也均具有抗HCV NS3蛋白酶抑制活性[6]。
从葡萄根提取物中得到的一种白藜芦醇四聚体vitisin B(11)显示出对HCV复制的抑制作用(IC50=6 nmol/L),而且细胞毒性较低(EC50>10 μmol/L)。与NS5B聚合酶抑制剂索磷布韦(sofosbuvir)联合使用时,vitisin B显示出协同作用。对vitisin B耐药的HCV变异株研究表明,NS3解旋酶为其潜在靶标。vitisin B可以和纯化的NS3解旋酶在体外直接结合,而显示出强效HCV NS3解旋酶抑制作用(IC50 = 3 nmol/L)。进一步的体内药动学实验研究表明,腹腔注射vitisin B后肝脏可以达到临床上可接受的浓度[7]。
Wu等[8]报道了从楝科植物大叶桃花心木(Swietenia macrophylla)枝干中分离得到化合物3-hydroxy caruilignan C (3-HCL-C, 12) 在蛋白和RNA水平均显示出较强的抗HCV活性,EC50 = 10.5 μmol/L。3-HCL-C与IFN-α、NS5B聚合酶抑制剂NM-107(2′-C-methylcytidine),或者NS3/4A蛋白水解酶抑制剂特拉匹韦联合使用,可以显著增强对HCV的RNA复制的抑制作用。
文献报道[9]五环三萜中的齐墩果酸(13)和熊果酸(14)能显著抑制HCV复制,IC50分别为2.9、10.6 μg/ml。而且这种作用部分是通过非竞争性抑制NS5B依赖性RNA聚合酶而产生。
从粘杆菌的次生代谢产物中得到的化合物soraphen A(15),具有显著的抗HCV活性,EC50为2.30 nmol/L。soraphen A既不抑制HCV RNA翻译,也不抑制HCV入侵,而是抑制HCV复制,可能是通过抑制HCV复制位点的膜质网发挥作用[10]。
Hu等从美洲小白菊(Parthenium hispitum Raf)中分离鉴定了9个C14-氧代1α-羟基-11(13)-伪愈创木烯6β,12-内酯类化合物,显示出较好的抑制HCV复制的作用[11]。其中化合物16在2 μmol/L浓度对HCV的抑制率可达90%。
-
HCV入侵正常肝细胞,是HCV完成感染正常肝细胞的最初阶段。如果可以阻止病毒对肝细胞的入侵,那么就可以将HCV“拒之门外”,从而起到保护正常细胞的作用。由于大多数药物开发策略都针对病毒生命周期的复制阶段,因此对于研究HCV入侵抑制剂是抗HCV药物的研究重要方向之一(图2),尤其是在接受肝移植的患者中。
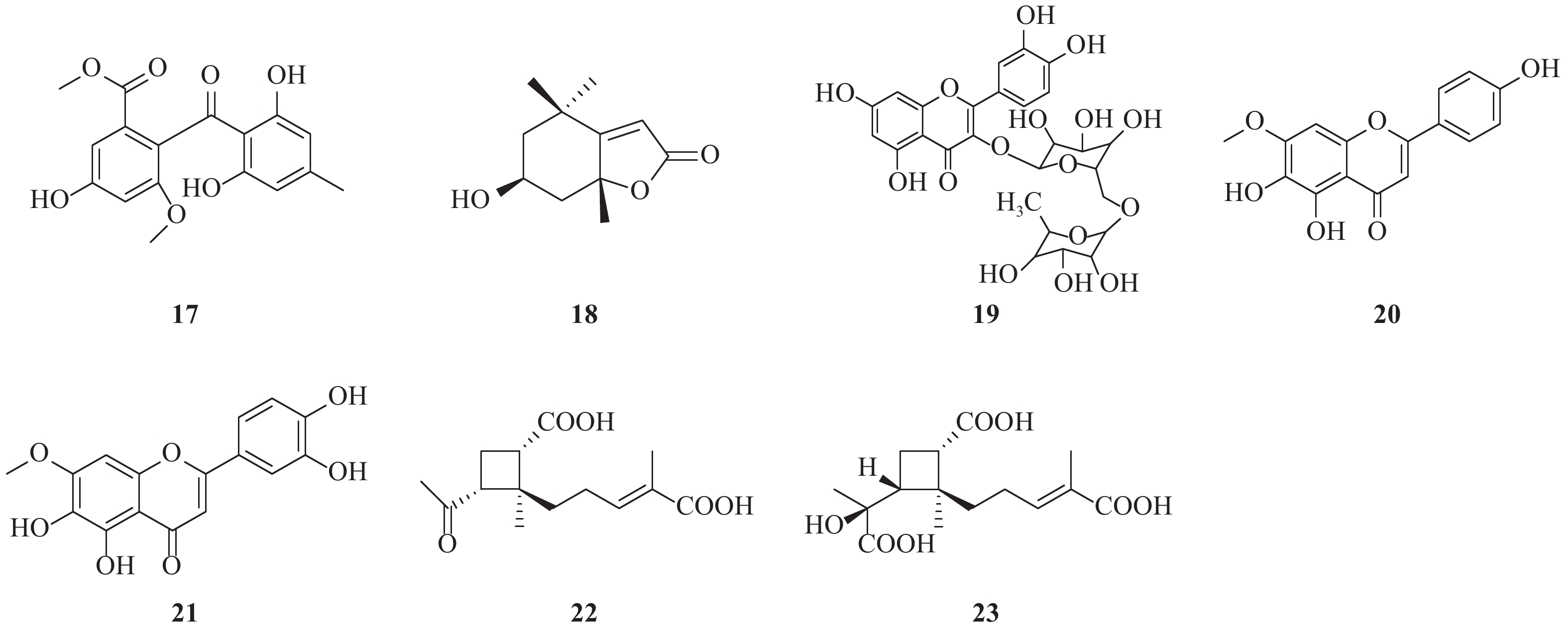
Nakajima等[12]从海藻、苔藓等分离出的真菌菌株的代谢产物中,发现了sulochrin(17)具有较好的抑制HCV活性。sulochrin是一种funicone类衍生物,可以以剂量依赖关系降低HCV对细胞的感染率和HCV核心蛋白的表达,与空白对照相比,可以降低至其1/3~1/4水平。而且,在浓度达50 μmol/L时,仍没有表现出明显的细胞毒性。进一步采用HCVpp进行实验,结果表明sulochrin可以抑制病毒入侵正常细胞。sulochrin与干扰素或者特拉匹韦联合使用,可以显著增强对抗HCV作用。构效关系研究表明,1,3-二羟基-5-甲基苯基结构是重要的药效基团。
从植物叶下珠(Phyllanthus urinaria)分离得到的一种单萜类内酯地芸普内酯(LOD, 18)是一种新型HCV抑制剂。LOD可以有效抑制有利病毒颗粒,抑制病毒黏附和阻止病毒入侵和融合,而对病毒复制或翻译影响以及I型IFN宿主抗病毒免疫反应影响很小。基于酶联免疫吸附测定(ELISA)的结合分析,证实了单萜类化合物能够有效阻断HCV颗粒黏附于宿主细胞表面的能力。此外,LOD可以抑制几种HCV基因型菌株的感染。LOD可能成为抗HCV药物的潜在候选药物[13]。
Qian等[14]研究发现从五味子中分离得到的五环三萜酸甘五酸(SZA)能有效抑制HCV入侵肝细胞,却不会干扰病毒在细胞表面的结合或内在化。但是,在SZA的作用下,细胞融合过程会受到损害,并且宿主膜的流动性增加。此外,还发现SZA可抑制HCV向邻近细胞的扩散,并且SZA与干扰素或特拉普韦的联合使用对抗HCV活性具有协同效应。除此之外,SZA能够抑制体内HCV感染。SZA靶标不同于常规的抗病毒药物。因此,SZA是一种用于HCV抑制剂的潜在治疗性化合物,尤其是对于在肝移植过程中需要预防HCV再感染的患者。
Bose等[15]以保肝作用闻名的水果为研究对象,从中筛选寻找HCV入侵抑制剂,发现蔷薇科李属植物欧洲李中分离得到的黄酮类化合物芦丁具有抗HCV作用。研究表明,芦丁(19)能够抑制HCV-LP(丙型肝炎病毒样颗粒)与肝癌细胞的结合,并抑制细胞培养来源的HCV(HCVcc)进入肝癌细胞。重要的是,芦丁对肝癌细胞没有毒性。此外,芦丁可以直接作用于病毒颗粒来抑制HCV生命周期的早期侵入阶段。因此,芦丁是一种用于治疗HCV感染的候选药物。
从南美植物Pterogyne nitens中分离得到了两种具有抗HCV作用的类黄酮化合物:对HCV复制周期有影响的珍珠梅素(sorbifolin, 20)和胡麻黄素(pedalitin, 21)。化合物20和21能够在非细胞毒性条件下抑制病毒进入高达45.0%和78.7%。胡麻黄素通过对病毒颗粒的直接作用和干扰,阻止病毒进入宿主细胞。同样,珍珠梅素也有抗HCV作用[16]。
从海绵真菌哈茨木霉菌(Trichoderma harzianum)中分离出两个新型倍半萜烯的类似物:哈兹烷酸A(harzianoic acids A, 22)和B(harzianoic acids B, 23)。 两种化合物均显示出降低HCV RNA水平的抑制活性,且细胞毒性较低。哈兹烷酸A的EC50=24.5 μmol/L,CC50=75.3 μmol/L;哈兹烷酸B的EC50=20.4 μmol/L,CC50=74.3 μmol/L。作用机制表明,这些化合物可以阻断HCV生命周期的侵入,而病毒E1/E2和宿主细胞CD81是潜在的靶蛋白[17]。
-
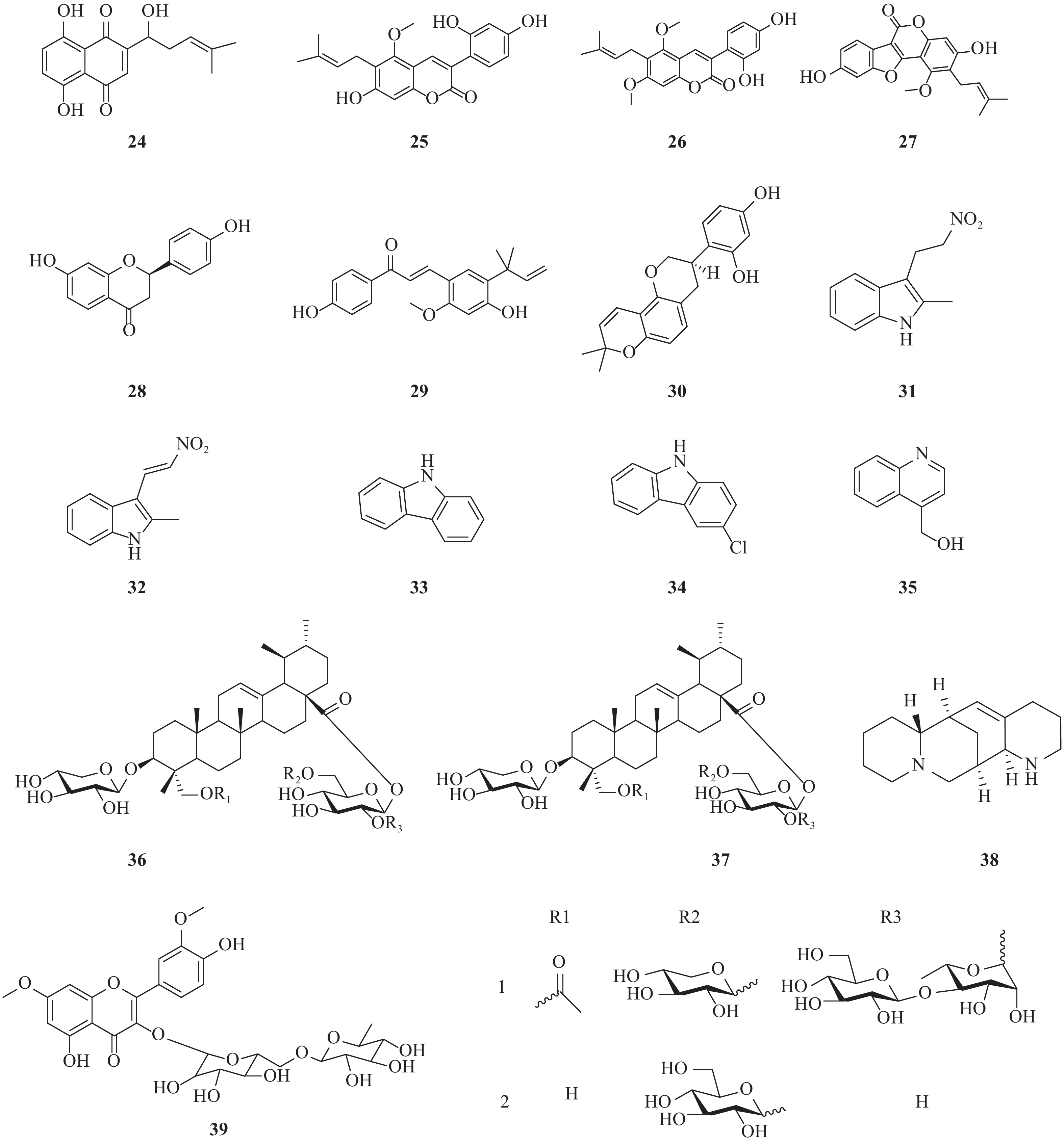
近年来,除了报道了一些已经初步明确了作用阶段或机制的天然产物外,还报道了一些未明确阶段和机制的化合物(图3)。Li等报道了从新疆紫草(Arnebia euchroma)分离得到的紫草素(24)显示出较好的抗HCV作用,其EC50= 0.025 μg/ml,CC50= 1.089 μg/ml,选择指数(SI)= 43.56[18]。
Adianti通过活性导向分离法从甘草中提取出的甘草香豆素(25)、甘草宁(26)、甘草酚(27)、甘草素(28)均具有较好抗HCV活性,IC50分别为8.8、7.2、4.6和16.4 mg/ml。但是,甘草的主要化学成分甘草酸以及甘草酸铵仅具有很弱的抗HCV活性。此外,甘草查耳酮A(licochalcone A)和光甘草定(glabridin)也显示出较好抗HCV活性,IC50分别为2.5和6.2 mg/ml。另一个查耳酮异甘草素也显示出抗HCV活性,IC50为3.7 mg/ml[19]。
由黏杆菌Labilithrix luteola(DSM 27648 T)分离而来的两种新型次生代谢产物,labindole A [2-甲基-3-(2-硝基乙基)-3H-吲哚, 29]和labindole B [2-甲基-3-(2-硝基乙烯基)-3H-吲哚, 30],以及已知的5种代谢物经生物活性检测发现:-氯-9H-咔唑(34)具有显著的抗HCV活性。9H-咔唑(33)和labindoles A(31)和B(32)都具有一定的HCV抑制作用,其中4-羟甲基喹啉(35)活性较弱,并且均没有细胞毒性[20]。
两种新型的乌苏烷型三萜皂苷bodiniosides O(36)和bodiniosides P(37)在体外均表现出一定的抗HCV活性,选择性指数分别为30.63和9.08。在所测试的化合物中,化合物36表现出一定抗HCV活性,EC50值为0.41 nmol/L,CC50值为12.56 nmol/L,SI值为30.63。此外,化合物37也具有较好的抗HCV活性,EC50值为1.58 nmol/L,CC50值为14.35 nmol/L,SI值为9.08[21]。
从豆科槐属植物苦豆子(Sophora alopecuroides)中提取的aloperine(38)是一种具有独特的内环结构的喹喔啉生物碱。研究发现其具有一定的抗HCV活性,最大有效浓度(EC50)为4.32 μmol/L,选择指数(SI)为54.5[22]。
从sarcocornia甲醇水提物中分离出一种新的黄酮醇三糖苷,鼠李糖3-O-2G-鼠李糖苷或鼠李糖苷3-O-(2″,6″-O-α-二鼠李糖基)-β-葡萄糖苷(39)。鼠李糖苷三糖苷被证实具有显著的抗HCV活性,IC50值为8.9 μmol/L[23]。
-
综上所述,近年来从天然产物中发现了大量的抗HCV活性结构,结构类型主要包括黄酮、萜类、木质素等。这些活性天然产物可以干扰HCV复制,抑制病毒入侵等,并且毒性较低。因此,从天然产物中寻找和发现的抗HCV活性结构对于抗HCV药物的研究具有重要意义。








 DownLoad:
DownLoad: