-
癌症对人类是仅次于心血管疾病的第二大“杀手”,严重威胁着人类的生命健康[1]。相关统计表明,2018年,全球新增癌症病例1 810万,死亡病例960万[2-3]。预计到2030年,新增癌症病例和死亡人数将增长约1.5倍,其防治形势十分严峻[4]。目前,治疗恶性肿瘤的方法主要有手术切除、放射疗法、生物治疗以及药物治疗等[5]。其中,药物治疗仍是肿瘤治疗的重要手段。但一半以上的癌症对传统化疗药物已产生明显耐药。临床证据显示,90%以上转移性癌症患者死于不同程度的耐药[6],肿瘤耐药已成为目前临床化疗失败的首要原因。因此,开发多药耐药的新型抗肿瘤化疗药物已迫在眉睫。
微管蛋白抑制剂一直是抗肿瘤药物研发的热点之一[7]。微管是由α/β微管蛋白组成的异二聚体,是细胞骨架的主要组成部分[8]。微管具有多种生物学功能,能参与细胞增殖、分裂等一系列过程[9-13],在细胞生命周期中起重要作用,是肿瘤细胞中的重要治疗靶标。紫杉醇、长春碱等微管蛋白抑制剂已广泛用于治疗多种癌症。然而,它们显示出治疗窗窄、选择性差以及多药耐药性问题,通常是由于P糖蛋白[14]或微管相关蛋白的过表达引起的[15]。因此,选择性好的低毒小分子靶向微管蛋白抑制剂仍有很大需求。
查尔酮衍生物是类黄酮中一类重要的化合物,分子结构简单,以1,3-二苯基丙烯酮为基本骨架,具有广泛的生物学活性,如抗肿瘤、抗氧化、抗炎、降血糖、抗菌等[16-19]。吲哚查尔酮是本课题组研究发现的一类新型查尔酮衍生物,相比经典的查尔酮,其抗肿瘤活性显著提高,作用机制研究表明该类化合物作用于β微管蛋白上的秋水仙碱位点[20],规避了P糖蛋白调控的肿瘤多药耐药机制[21]以及β-Ⅲ型微管蛋白过表达的影响[22],具有良好的抗肿瘤耐药活性。化合物FC58是本课题组前期合成的查尔酮类衍生物之一,具有非常好的抗肿瘤活性(A549,GI50 = 38 nmol/L)[23]。课题组以此化合物为研究对象,考察其对多种白血病细胞株的生长抑制活性,特别是耐药白血病细胞的活性,并通过细胞周期实验分析其作用特点,现报道如下。
HTML
-
全自动酶标仪(BioTek,型号:Synergy 2);流式细胞仪(BD,型号:Accuri C6);熔点测试仪(上海精科,型号:WRR);核磁共振仪(Bruker,型号:Ascend 400);高分辨质谱仪(Waters,型号:UPLC G2-XS Q-TOF);薄层色谱硅胶板(烟台江友,型号:HSGF254)。实验所用试剂均为分析纯(伊诺凯)。紫杉醇、长春碱、多柔比星(Sigma-Aldrich),纯度>98%。HL60、HL60/DOX、K562、K562/HHT300、CCRF-CEM、CCRF-CEM/VLB100由海军军医大学药学院天然药物化学教研室传代和冻存。
-
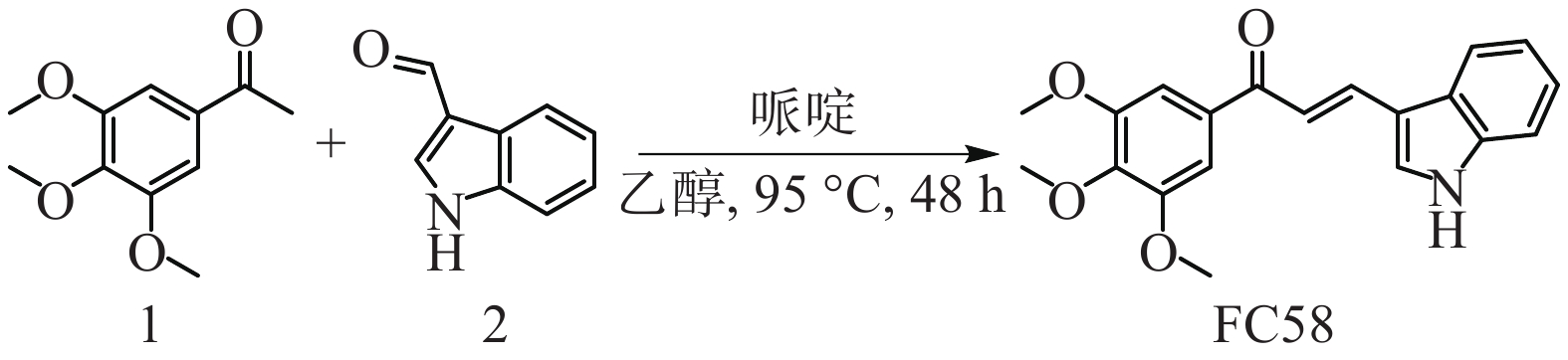
将1-(3,4,5-三甲氧基苯基)乙-1-酮(1, 0.21 g, 1.0 mmol)与1H-吲哚-3-甲醛(2, 0.07 g, 0.5 mmol)溶于乙醇(4 ml)溶液中,加入哌啶(0.11 ml, 1.2 mmol),加热至95 ℃搅拌48 h,然后加入盐酸调节pH = 6淬灭反应,用乙酸乙酯萃取,饱和碳酸氢钠溶液、水和饱和氯化钠溶液洗涤,无水硫酸钠干燥、过滤、旋干有机相,剩余粗品经乙醇在−20 ℃重结晶,得到目标化合物FC58 0.017g,重结晶产率:10%,黄色固体,熔点:175.1~176.8 ℃,路线见图1。1H NMR (400MHz, CDCl3) δ : 8.71 (1H, br, NH), 8.13 (1H, d, J = 15.6 Hz, =CH), 8.00 (1H, m, Ar-H), 7.63 (1H, s, Ar-H), 7.55 (1H, d, J = 15.2 Hz, =CH), 7.46 (1H, m, Ar-H), 7.31 (4H, m, Ar-H), 3.96 (9H, s, 3×OCH3)。13C NMR (100MHz, CDCl3) δ : 189.86, 153.10, 138.78, 137.23, 134.44, 130.08, 123.57, 120.54, 117.82, 114.52, 111.99, 105.96, 60.97, 56.40。ESI-MS (m/z) : C20H19NO4 [M+H]+,理论值:338.1387, 实际值:338.1385。
-
样品配制:用适量DMSO(Sigma,C6295)溶解后,配成50 mmol/L的母液备用。取一定量的母液,用完全培养基将其稀释成一定浓度的溶液,作为初浓度。将初浓度溶液用完全培养基3倍稀释,共设置6个浓度梯度。
CellTiter-Blue法测试体外抗肿瘤活性。具体方法简述如下:96孔板每孔加入浓度为(2~4)×104个/ml的细胞悬液200 μl,置37 ℃,5% CO2培养箱内。24 h后,加入配制好的一定浓度梯度的待测物溶液,每孔200 μl,设置3个复孔,空白对照组加入200 μl的含1% DMSO的完全培养基,置于37 ℃,5% CO2的培养箱内48 h。每孔加入含10% CellTiter-Blue(Promega,G8081)的完全培养基,用全自动酶标仪测530/40 nm和590/35 nm处的荧光值(FV)作为背景值。置于37 ℃、5% CO2的培养箱内1~4 h,再用酶标仪在相同条件下读取荧光值。
细胞存活率(%)= 给药孔∆FV/空白对照孔∆FV。
根据各个浓度的细胞存活率值,使用GraphPad Prism 5软件对数据进行拟合可得到量效曲线和抑制细胞生长50%的药物浓度,即GI50。
根据药物对非耐药母代细胞和对耐药细胞的GI50计算药物的耐药指数(DRI)。公式: DRI =GI50(耐药细胞)/GI50(母代细胞)。
-
取对数生长的HL60和HL60/DOX细胞,用胰酶消化分散成单细胞悬液,以(5×105个/孔)接种于96孔板中,于37 ℃、5% CO2的培养箱中培养24 h。加入不同浓度的待测药物继续培养24 h。弃上清液,用0.25% Trypsin-EDTA(Gibco,25200056)消化,1000 r/min离心5 min收集细胞。用1 ml预冷的PBS(Hyclone,SH30256.FS)润洗细胞1次,离心收集细胞。细胞沉淀用9 ml预冷的70%乙醇轻轻混匀,4 ℃固定过夜。次日,离心沉淀细胞后,弃固定液,用1 ml预冷的PBS洗涤细胞2次,再次离心收集细胞。加入1 ml PI(50 μg/ml PI, 0.1 mg/ml RNase A, 0.05 % TritonX-100,碧云天)染液染色,4 ℃下避光孵育30 min,流式细胞仪检测,激发波长为488 nm,用EXPO32 ADC软件进行分析。
2.1. (E)-3-(1H-吲哚-3-基)-1-(3,4,5-三甲氧基苯基)丙-2-烯-1-酮的合成[24]
2.2. 体外抗肿瘤活性测试[23]
2.3. 细胞周期试验[23]
-
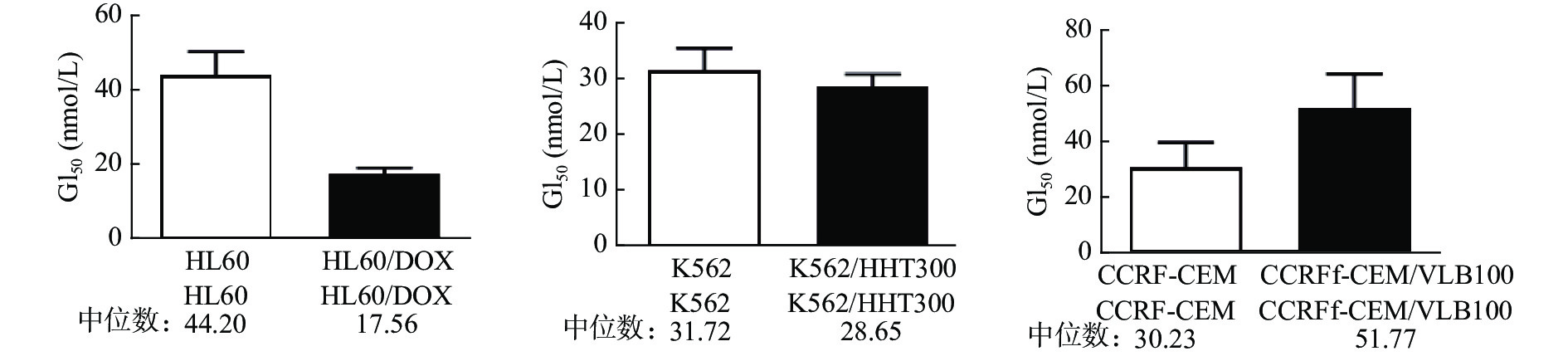
课题组选择多种白血病细胞及其耐药细胞(HL60、HL60/DOX、K562、K562/HHT300、CCRF-CEM、CCRF-CEM/VLB100)对紫杉醇、长春碱、多比柔星以及查尔酮衍生物FC58进行体外抗肿瘤活性测试。结果如表1所示,紫杉醇、长春碱和多柔比星对HL60、K562、CCRF-CEM细胞均有较强活性,GI50在0.12~135.30 nmol/L。而各耐药细胞对上述3种药物明显耐药,GI50在17.16~1398 nmol/L。耐药指数DRI在3.85~684.4。与紫杉醇、长春碱、多柔比星相比,虽然查尔酮衍生物FC58对以上白血病细胞的活性相对较弱,GI50在30.23~44.20 nmol/L,但对耐药白血病细胞具有优异活性,GI50在17.56~51.77 nmol/L,较亲代细胞更为敏感(图2)。DRI分别是0.40、0.90、1.71。其中,CCRF-CEM/VLB100细胞对FC58仅轻微耐药(DRI=1.71),远低于其他几种化疗药。
细胞系 化合物 紫杉醇 长春碱 多比柔星 FC58 HL60 6.68 2.90 40.27 44.20 HL60/DOX 292.50 89.52 1398 17.56 耐药指数 43.79 30.87 34.72 0.40 K562 12.15 0.12 135.30 31.72 K562/HHT300 98.31 17.16 521.40 28.65 耐药指数 8.09 143 3.85 0.90 CCRF-CEM <1 <1 − 30.23 CCRF-CEM/VLB100 159.3 684.4 − 51.77 耐药指数 >159.3 >684.4 − 1.71 注:“−”表示未检测。 -
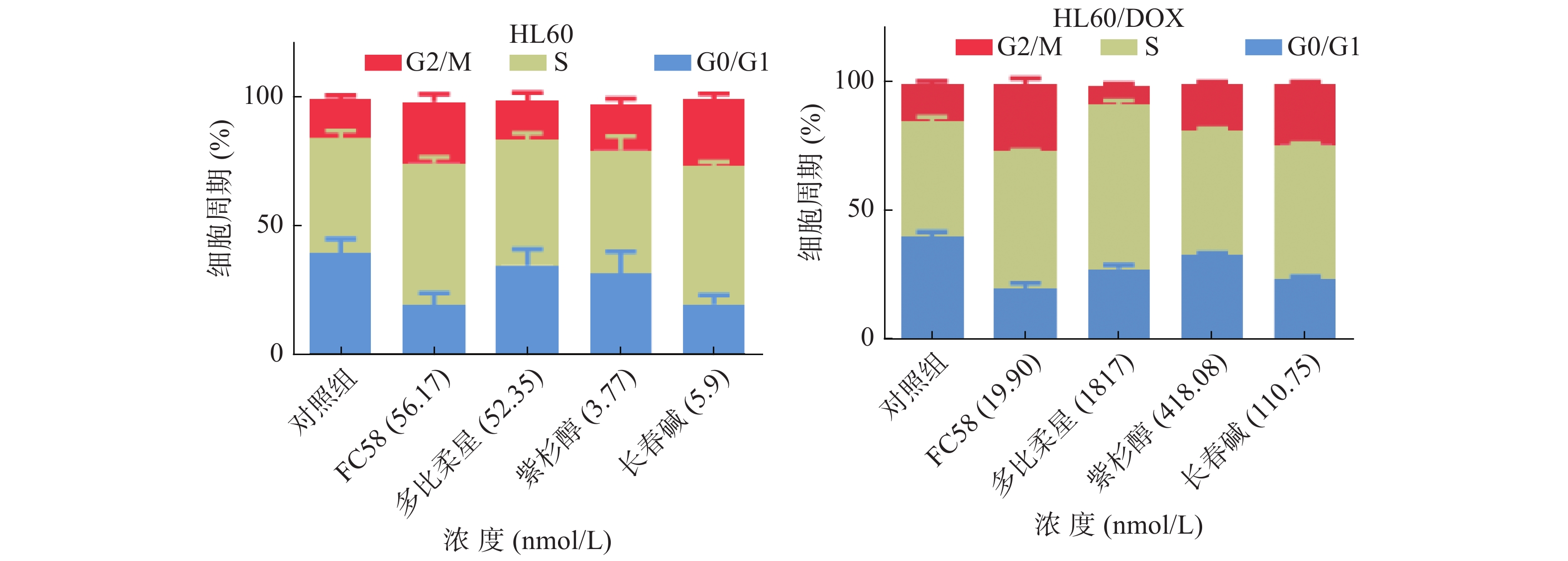
微管蛋白抑制剂可干扰微管蛋白-微管之间的平衡,阻断有丝分裂M期纺锤体的形成,导致有丝分裂停滞于G2/M期,从而诱导细胞凋亡[25]。利用流式细胞技术检测FC58、多柔比星、紫杉醇和长春碱对HL60和HL60/DOX细胞周期的影响,考察其作用机制。
如图3所示,与空白组相比,经1.3倍IC50 FC58处理的组,停滞在G2/M期的细胞明显增多。其作用强于紫杉醇,弱于长春碱。经多柔比星处理的组,停滞在S期和G2/M期的细胞较空白组有一定程度的增多。FC58、紫杉醇和长春碱的耐药细胞组的结果与非耐药细胞组结果基本相同。经FC58处理的组,停滞在G2/M期的细胞明显增多,且作用强于其余药物。多柔比星使细胞周期停滞在S期的耐药细胞明显增多。上述结果提示,FC58的作用靶点为微管蛋白。同时,FC58对细胞周期的影响结果与长春碱较相似,间接证明了FC58和长春碱作用机制相似。这可能是FC58对CCRF-CEM/VLB100细胞轻微耐药的原因。
3.1. 体外抗肿瘤活性研究
3.2. 细胞周期分析
-
吲哚查尔酮类衍生物FC58对多种白血病细胞均有较强活性,GI50达纳摩尔级。对耐药的白血病细胞同样具有较强的活性,比亲代白血病细胞更为敏感,活性抗耐药指数远强于紫杉醇、长春碱和多柔比星。FC58和其他微管蛋白抑制剂一样都能使细胞周期停滞在G2/M期,间接验证其作用靶点为微管蛋白。FC58作为一个潜在的抗耐药白血病的先导化合物,有必要进一步研究其体内的抗肿瘤活性。









 DownLoad:
DownLoad: