-
盆腔炎是一种常发病于年轻女性上生殖道感染的妇科疾病。临床研究发现,盆腔炎患者上生殖道内存在大量激活的巨噬细胞[1]。在炎症和病原体的刺激下,巨噬细胞过度激活可以释放出大量炎症因子,包括肿瘤坏死因子-α(TNF-α),白细胞介素-1β(IL-1β)和白细胞介素-18(IL-18)。研究发现,NLRP3炎性小体激活可以促使caspase-1活化并切割IL-18、IL-1β前体,促进IL-18、IL-1β的成熟与释放,而抑制NLRP3炎性小体过度激活以减轻盆腔炎临床症状,并且与降低炎症因子、趋化因子释放有关。巨噬细胞在不同刺激下可以活化为不同表型:经典活化的M1型和替代活化的M2型。在含有脂多糖(LPS)和IFN-γ微环境中,巨噬细胞活化为变形虫样的M1型,参与炎症的发生。巨噬细胞在含有IL-4、IL-10、IL-13等抗炎因子微环境中被活化为M2型,表达精氨酸酶 1(Arg-1)和甘露糖受体1(CD206) 等特异性标志分子,参与炎症消退和组织重塑。研究发现,M1 /M2比例失衡是多种炎症性疾病的病理标志,如肥胖[2]、糖尿病[3]、动脉粥样硬化[4]等。
益母草碱(LEO)是一种具有抗炎、抗氧化和抗肿瘤作用的天然黄酮类化合物[5],研究报道显示益母草碱可抑制Bax/Bcl-2信号通路激活抑制炎症因子的表达[6]。但鲜有益母草碱对巨噬细胞中NLRP3炎症小体影响的报道。本实验以益母草碱为研究对象,探讨其对巨噬细胞中NLRP3炎症小体激活的影响,以及对巨噬细胞M1/M2表型的调节作用。
HTML
-
益母草碱(纯度>98%,西格玛奥德里奇贸易有限公司,上海);脂多糖(L6143)、DMEM高糖培养基、胎牛血清、NuPAGE 10% Bis-Tris Gel、10×MOPS SDS 运行缓冲液、10×传输缓冲液、预制蛋白Marker、荧光定量PCR、反转录试剂盒(美国赛默飞世尔科技公司);青-链霉素混合液、胰蛋白酶(美国Hyclone公司);Griess试剂盒(江苏碧云天生物试剂公司);IL-1β、IL-18、IL-6、TNF-α等ELISA试剂盒(武汉伊莱瑞特生物公司);PVDF膜(美国Millipore公司);caspase-1兔抗单克隆抗体、β-actin兔抗单克隆抗体、羊抗兔/羊抗鼠单克隆二抗(武汉三鹰生物技术有限公司);NLRP3兔抗单克隆抗体(英国Biorbyt生物试剂公司);四甲基偶氮唑蓝溶液(MTT,美国Bio-Rad公司);TRIzol Regent试剂盒(日本TaKaRa公司)。
-
C57BL/6小鼠,6周龄,雌性,体重(20±2) g,购于江苏集萃药康生物科技股份有限公司。小鼠饲养于实验室SPF级动物房,温度(22 ± 1)°C和湿度(60 ± 2)%,动物自由饮食。动物实验操作均通过实验动物伦理委员会批准。
-
以颈椎脱臼的方式处死小鼠,置于75%的乙醇溶液中浸泡10 min,将15 ml PBS缓冲液注入小鼠腹中,仰卧平放,揉捏小鼠腹部5 min,吸出腹液,离心分离巨噬细胞,用DMEM培养液调整细胞浓度,在细胞培养箱中以5%CO2、37 ℃恒温孵育24 h后,换液,去除未贴壁细胞,即得到纯化的小鼠腹腔巨噬细胞[7]。以1.5×105个/ml密度接种于24孔(或96孔)板中培养24 h后,随机分为空白组、益母草碱(10 μmol/L)组、脂多糖(1 μg/ml)组、脂多糖+益母草碱(10 μmol/L)组,益母草碱预处理1 h之后加入脂多糖,放回孵箱中培养,24 h后,提取上清液和细胞蛋白,检测相关指标。
-
脂多糖预处理巨噬细胞24 h后,在96孔板中每孔加入10 μl(5 mg/ml)MTT溶液,于37 ℃孵箱中培养,4 h后取出,移除细胞上清液,每孔加入200 μl二甲基亚砜,放置于恒温摇床震摇10 min,于562 nm处测定OA值,检测相关指标。
-
Griess试剂盒取出,恢复至室温。将细胞上清液和标准品和加入到96孔板中,将试剂I 和试剂II 混匀加入96孔板中,避光,放置于恒温摇床震摇30 min,于470 nm处测定OA值,检测NO含量。
-
提前将ELISA试剂盒取出,恢复至室温。稀释细胞上清液,配置标准品工作液,按照ELISA试剂盒说明书要求依次加入反应液,最后加入终止液,于450 nm处检测OD值,计算IL-1β、IL-18、IL-6、TNF-α含量。
-
按照Trizol法提取巨噬细胞中总RNA,根据逆转录试剂盒说明书,进行RT-PCR反应。设定反应条件为:5 ℃预变性30 s,接着95 ℃变性6 s,最后60 ℃退火,延伸37 s,重复反应40个循环。RT-PCR引物设计见(表1)。以GAPDH为内参,利用2−∆∆Ct方法分析结果。
基因 引物序列(5′→3′) NLRP3 F: AGAAGAGACCACGGCAGAAG R: CCTTGGACCAGGTTCAGTGT ASC F: TGGATGCTCTGTACGGGAAG R: CCAGGCTGGTGTGAAACTGAA caspase-1 F: CTTGGAAATAGCTCCCAGAA R: CATTTGGGAACTTCTCATCC TNF-α F: CCAATGGCAGAGTGGGTATG R: TGAAGAGGACCTGGGAGTAG iNOS F: GGGAATCTTGGAGCGAGTTG R: GTGAGGGCTTGGCTGAGTGA CD206 F: CAGGTGTGGGCTCAGGTAGT R: TGGTGAGCTGAAAGGTGA Arg-1 F: TTGCTGTGCTCCATAGTTTCCA R: CCATGCAAGTTTCCACTTGT GAPDH F: GGAGAAACCTGCCAAGTATG R: TTACTCCTTGGAGGCCATGTAG -
脂多糖处理巨噬细胞24 h后,弃去细胞上层培养基,置于冰上,PBS洗涤3次,加入RIPA裂解液(含1%PMSF)反应30 min,离心,收集上清液。BCA蛋白定量试剂盒检测蛋白含量,配置缓冲液,变性。每孔10 μl加入到10%预制胶中,设置电压200 V电泳30 min,设置电压25 V电转30 min,TBST洗涤,5%脱脂牛奶封闭2 h,TBST洗涤,4 ℃一抗孵育过夜,TBST洗涤,二抗孵育30 min,TBST洗涤,加入曝光剂,曝光。
-
采用SPSS18.0分析实验中所涉及的数据,组间比较方差齐,用LSD检验,方差不齐采用 Dunnett’s T3检验,以P < 0.05为统计学差异,数据结果用均数 ± 标准误(
$\bar x \pm s$ )表示。
1.1. 实验材料
1.2. 实验动物
1.3. 细胞培养及给药
1.4. MTT检测细胞活性
1.5. Griess试剂盒检测NO含量
1.6. ELISA试剂盒检测IL-1β、IL-18、IL-6、TNF-α含量
1.7. 实时荧光定量(RT-PCR)实验
1.8. Western blot 检测蛋白NLRP3、ASC、caspase-1的表达
1.9. 数据统计
-
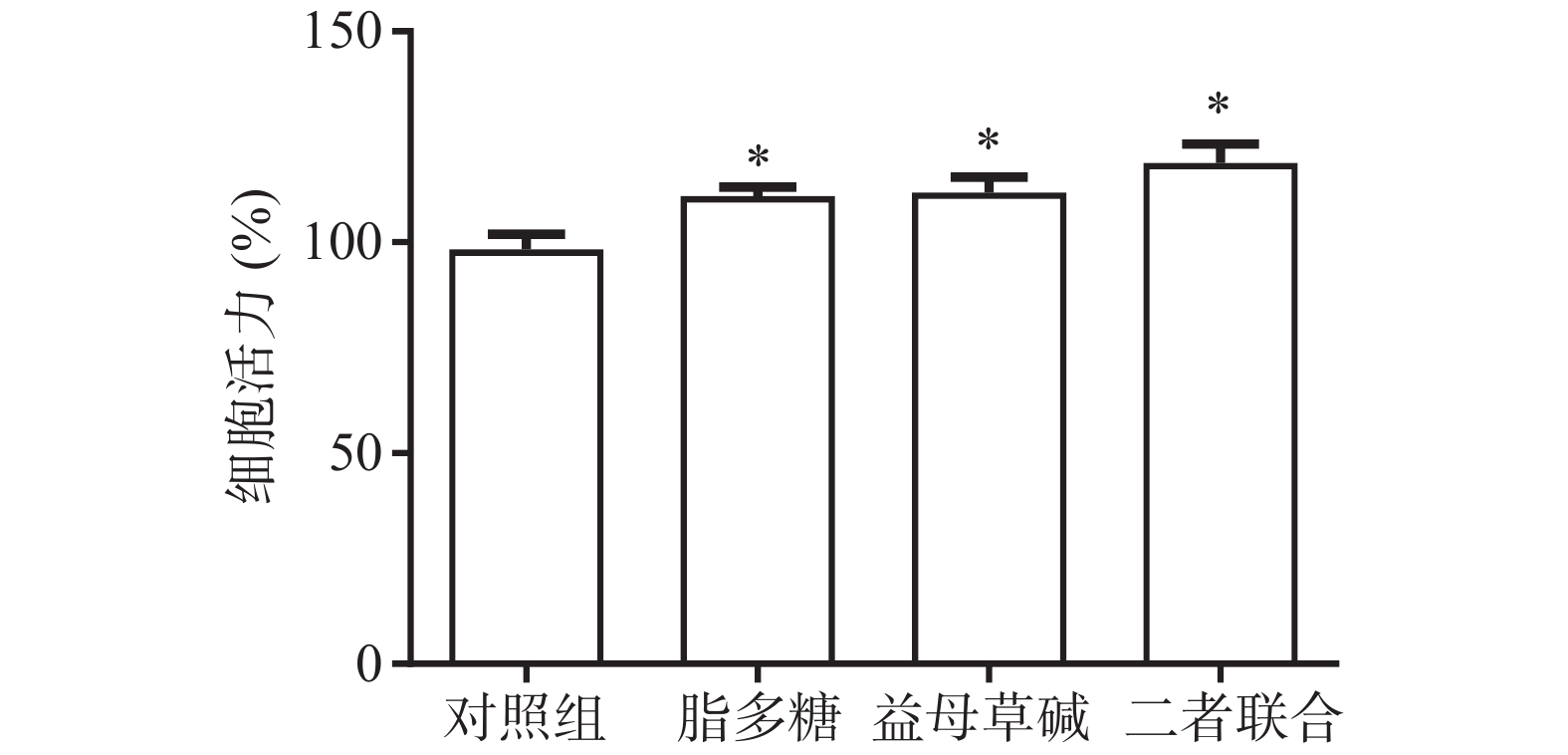
首先,观察益母草碱和脂多糖对巨噬细胞活力的影响(图1)。结果显示,脂多糖和益母草碱均能提高巨噬细胞的活力(P<0.05),当益母草碱与脂多糖共同刺激巨噬细胞时,巨噬细胞活力得到了进一步的增强(P<0.05)。
-
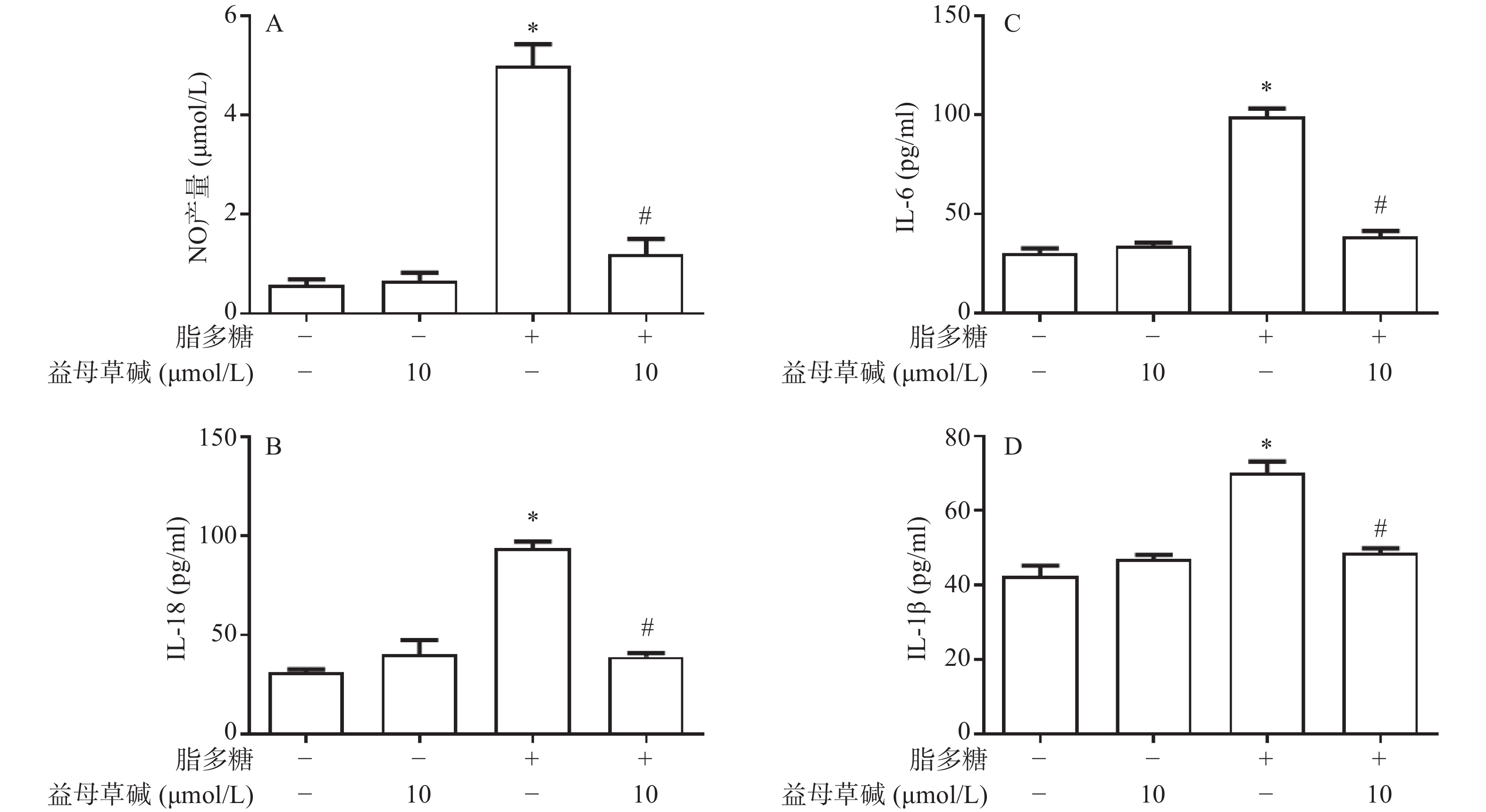
观察益母草碱对巨噬细胞炎症因子释放的影响,在脂多糖刺激下,巨噬细胞上清液中NO的释放增加,而益母草碱可以抑制巨噬细胞NO释放(图2A)。检测脂多糖对巨噬细胞上清液中IL-1β、IL-18和IL-6释放的影响,结果发现,益母草碱可以降低巨噬细胞IL-1β、IL-18和IL-6释放(图2B-2D)。结果显示,益母草碱可以减少脂多糖引起的巨噬细胞炎症因子的释放。
-
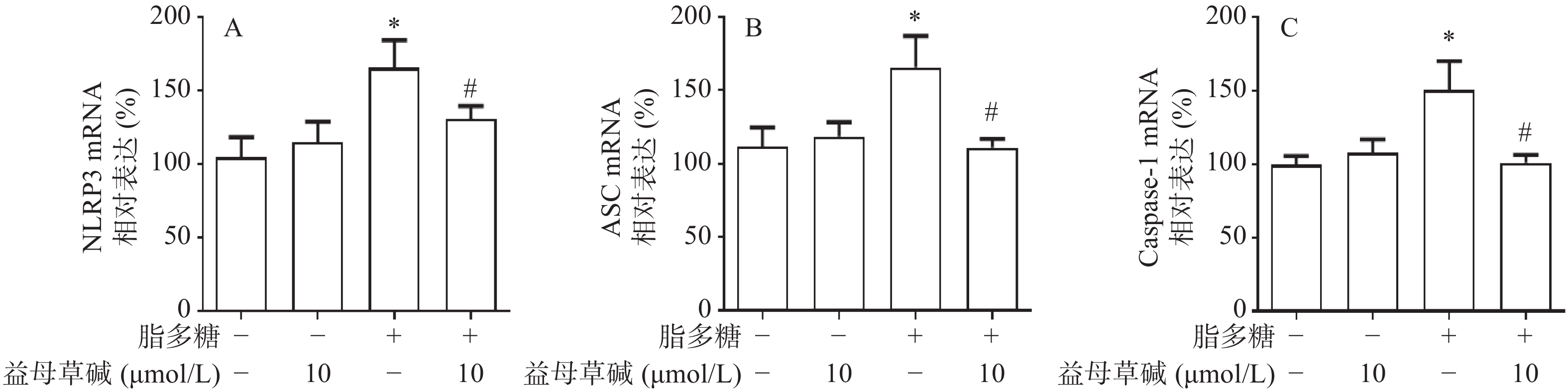
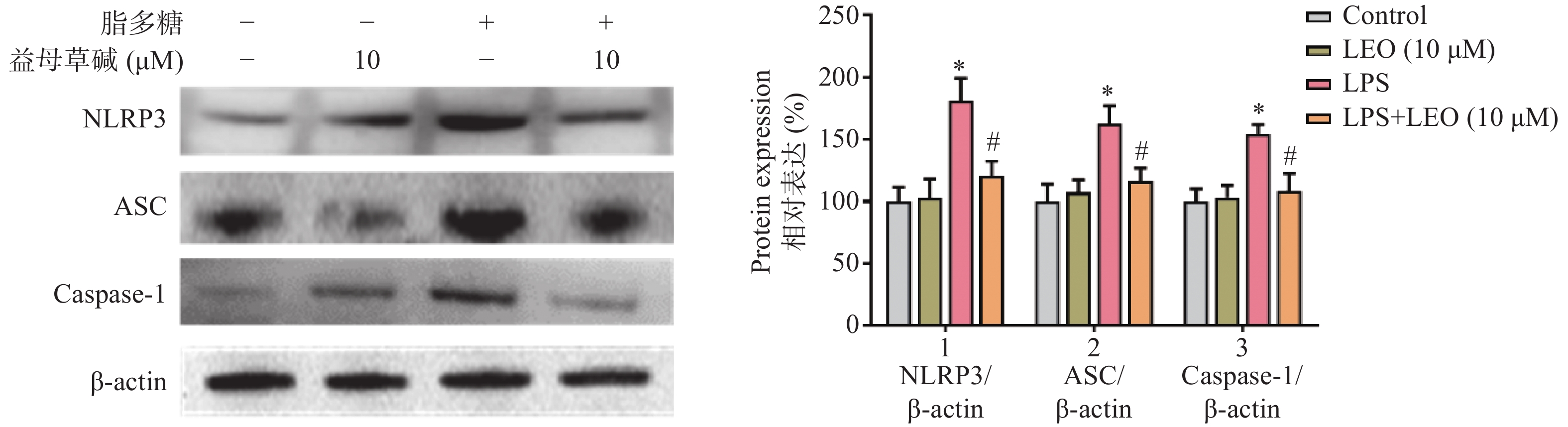
细胞内IL-1β、IL-18等炎症因子的释放需要经过NLRP3炎症小体的激活,为此,观察了益母草碱对NLRP3炎症小体激活的影响。RT-PCR结果显示(图3),脂多糖刺激后,NLRP3、ASC、caspase-1的mRNA表达增加,益母草碱可以降低mRNA表达。Western blot结果也证实益母草碱可以抑制脂多糖引起的巨噬细胞中NLRP3、ASC、caspase-1蛋白表达(图4)。
-
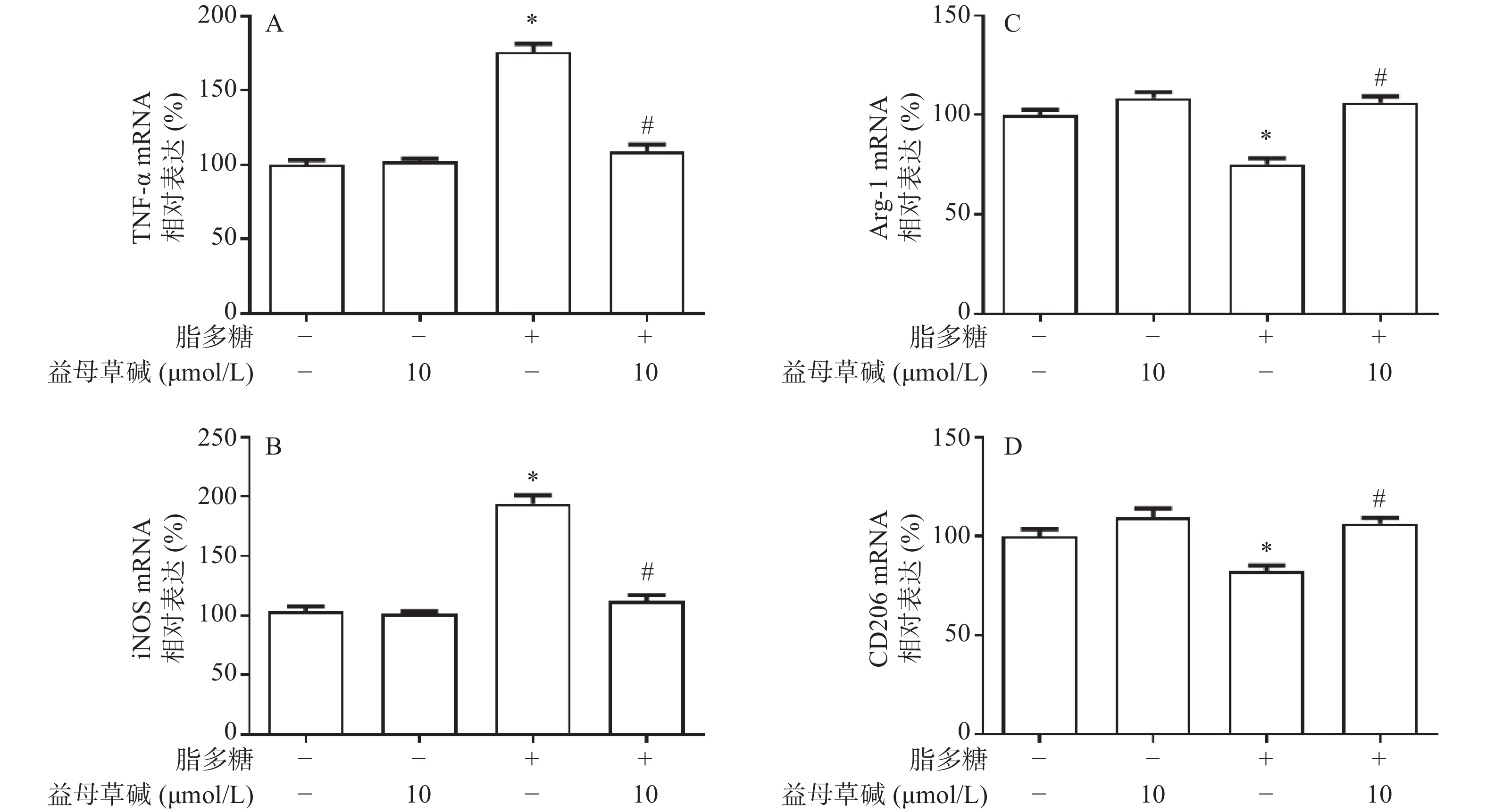
应用RT-PCR检测益母草碱对巨噬细胞M1/M2表型的影响。正常情况下,巨噬细胞M1型标志物TNF-α和iNOS表达量较低,经脂多糖诱导刺激后TNF-α和iNOS的表达水平显著升高,益母草碱可以降低脂多糖引起的TNF-α和iNOS的mRNA表达(图5A和5B)。另一方面,脂多糖诱导刺激后,巨噬细胞M2型标志物Arg-1和CD206的表达水平降低,益母草碱预处理可以增加脂多糖引起的Arg-1和CD206表达(图5C和5D)。表明益母草碱可以调控脂多糖引起的巨噬细胞由M1向M2型转化。
2.1. 益母草碱对巨噬细胞活力的影响
2.2. 益母草碱抑制巨噬细胞NO、IL-1β、IL-6及IL-18释放
2.3. 益母草碱抑制脂多糖引起的巨噬细胞中NLRP3炎症小体激活
2.4. 益母草碱调节巨噬细胞由M1型向M2型转化
-
研究发现在盆腔炎患者的上生殖道内存在大量激活的巨噬细胞[8]。巨噬细胞是参与炎症反应的天然免疫细胞,当病原体入侵或者组织发生病变时,巨噬细胞分泌多种炎症因子,诱导更多的巨噬细胞活化、募集,加强局部抗炎作用。正常生理情况下,炎症因子的含量极少,具有维持机体免疫和调节心脑血管等功能[9]。但当机体长期受到病原微生物、致炎因子刺激时,会导致一系列病理改变,如长期慢性子宫内膜炎刺激可以增加子宫纤维化的发病率[10]。基于此,本实验利用革兰阴性菌来源的脂多糖刺激巨噬细胞,观察益母草碱对脂多糖诱导的巨噬细胞激活和炎症因子表达的影响。结果显示,脂多糖刺激可以引起巨噬细胞过度激活,相关炎症因子表达增加,益母草碱预处理可以减少炎症因子的表达和分泌,提示益母草碱的抗炎作用与抑制巨噬细胞中炎症因子的产生有关。
为了进一步阐明益母草碱的抗炎作用,本实验对NLRP3炎症小体进行了研究。大量研究发现脂多糖可以激活NLRP3炎症小体,引起细胞因子释放增加。鉴于盆腔炎是炎性刺激引起的病理变化,且抑制NLRP3炎症小体可以降低炎症,推测抑制NLRP3炎症小体过度激活可能对盆腔炎起到一定的治疗效果。本实验中发现,益母草碱可以通过抑制脂多糖引起的巨噬细胞中NLRP3炎症小体相关蛋白表达,从而减少炎症因子释放,证实益母草碱可以抑制脂多糖诱导的巨噬细胞内NLRP3炎症小体的过度激活发挥抗炎作用。
巨噬细胞的表型转化在盆腔炎的病理进程中发挥着重要作用,M1型巨噬细胞主要发挥促炎、吞噬病原体的作用,M2型巨噬细胞主要发挥促进组织重塑、损伤修复等。因此,在盆腔炎疾病中,M1型巨噬细胞能够加重上生殖道炎症进展,而M2型巨噬细胞能抑制疾病进展。为了明确脂多糖对巨噬细胞分化的影响,使用RT-PCR实验验证不同处理方式对巨噬细胞分型的影响。结果显示,脂多糖能够促进M1型标志物TNF-α和iNOS的mRNA表达,而益母草碱能明显抑制 TNF-α和iNOS的mRNA表达,同时促进M2型标志物Arg-1和CD206的mRNA表达。上述结果提示,益母草碱能抑制脂多糖诱导的巨噬细胞向M1型分化以及IL-18、IL-1β、TNF-α表达,促进巨噬细胞向M2型分化。
在炎症反应过程中,脂多糖可以引起巨噬细胞中IL-1β、IL-18、IL-6、TNF-α等炎症因子表达增加,益母草碱可以通过抑制NLRP3炎症小体激活发挥其抗炎作用,提示抑制NLRP3炎症小体过度激活可能成为盆腔炎治疗的新策略,同时,本研究也为进一步开发益母草碱作为妇科用药提供理论基础。










 DownLoad:
DownLoad: