-
神经损伤是世界范围内致残和导致死亡的主要原因,神经损伤疾病患病率的大幅增加导致了全社会的健康负担加重[1]。神经损伤主要包括颅脑损伤(TBI)和缺血性脑卒中(CI)等,其中TBI是最常见的神经损伤类型[2],分为急性和慢性两个阶段,炎症是这两个阶段的共同特征,目前还没有有效治疗TBI的药物和方法,迫切需要寻找具有更广泛作用的药物,以缓解TBI后炎症反应[3]。脑缺血是神经损伤患者死亡的最常见病因之一[4],大脑因供血中断而致脑缺血,进而导致中风等神经损伤性疾病[5]。CI诱导了多种细胞死亡形式,如兴奋性毒性、酸毒性和离子失衡、氧化/氧化应激、炎症[6]、凋亡和梗死周围去极化等。目前CI最有效的治疗手段是静脉溶栓和血管内取栓以达到快速再灌注,这两者都能降低患者致残率,但都需要在发病4小时内尽快完成[4],目前尚无有效的药物治疗CI患者。
中药具有多途径、多靶点的特点,已有2000多年的发展历史和临床用药经验[7],如唐·孙思邈所著《备急千金方》中“小续命汤治卒中风欲死”和“大秦艽汤(金·刘完素)治中风”等。因此,阐明在临床上广泛使用的中药的作用机制是本文关注的重点。益母草来自唇形科植物益母草(Leonurus japonicus Houtt)的新鲜或干燥地上部分,临床上主要用于子宫收缩和镇静[8]。现代药理学研究发现其具有子宫收缩、抗炎、镇痛和抗氧化作用等[9]。值得引起关注的是,益母草对神经损伤也有保护作用[10]。然而,关于益母草治疗神经损伤的物质基础和作用机制的研究还未见报道。因此有必要阐明益母草治疗神经损伤的物质基础和作用机制。
本研究旨在利用网络药理学预测益母草的活性成分、靶点及相关通路来探讨其治疗神经损伤的潜在分子机制,为益母草的药理机制深入研究和临床应用提供参考。
-
中药系统药理学分析平台(TCMSP)和中药分子机制的生物信息学分析工具(BATMAN-TCM);活性成分靶标预测数据库(SwissTargetPrediction, STP);人类基因注释数据库(GeneCards);疾病靶点标准化数据库(Uniprot);京都基因与基因组百科全书; 蛋白-蛋白相互作用网络平台(STRING 11.0);Venny2.1软件、Cytoscape 3.6.0软件和在线作图工具微生信。
-
通过TCMSP和BATMAN-TCM数据库输入“yimucao”,搜索得到益母草的活性成分, 然后,在TCMSP中设置口服生物利用度(OB)≥30%及药物相似性(DL)≥0.18;在BATMAN-TCM中设置“药物-靶点”相似性模型阀值≥20,调节P值≤0.05,筛选活性成分。
-
在获得益母草活性成分的基础上,检索TCMSP和STP数据库,限定种属为“Homo sapiens(人类)”,获取活性成分的作用靶点。通过PubChem数据库来确证收集到的活性成分,将其标准化并下载 SMILES序列。再通过TCMSP数据库搜索确证后的活性成分的靶点,将搜索的靶点按照度值从大到小排列后得到益母草的潜在靶点。在数据库STP中,搜索SMILE式,筛选条件为“概率>0”,删除重复值后预测得到药物的潜在靶点。此外,由于益母草中葫芦巴碱已被证实具有较好的治疗神经损伤作用,故将该化合物也纳入活性成分范围内[11],并通过TCMSP和STP数据库获取其成分靶点。
-
通过GeneCards、DisGenet、OMIM数据库以疾病名称“cerebral ischemia”和“traumatic brain injury”进行检索,获得神经损伤相关靶标。
-
利用疾病靶点标准化数据库Uniprot,分别上传上述得到的益母草潜在靶点与神经损伤相关靶点名,获取其靶点的标准基因名以及Uniprot ID。为明确益母草治疗神经损伤疾病的潜在靶点,将二者的靶点上传至Venny 2.1 软件绘制韦恩图,并导出交集的基因。再将筛选得到的共有靶标蛋白上传至STRING平台,选择“multiple proteins”模式,建立药物靶蛋白-疾病靶蛋白相互作用网络,结合分值取中等“medium confidence(≥0.4)”,其余参数默认,利用 Cytoscape3.6.0软件构建益母草治疗神经损伤的PPI网络。利用cytoHubba插件计算 PPI网络每个节点的度值,筛选益母草治疗神经损伤的核心靶点。
-
在上述STRING中的结果下,选择“Analysis”,点击下载“Biological Process(GO)”“Molecular Function(GO)”“Cellular Component(GO)”“KEGG Pathways”。阈值设置为P≤0.01, 并按照涉及的靶点数目多少进行排序,得到GO气泡图和 KEGG 信号通路条形图。
-
通过TCMSP数据库检索到益母草已报道的化学成分,以ADME参数OB≥30%、DL≥0.18进行筛选,得到益母草活性成分8个;BATMAN-TCM数据库检索到10个益母草活性成分;加上文献检索的1个化合物共19个(表1)。再将这19个活性成分输入TCMSP以及STP数据库,搜索的结果经过筛选去除重复项后共得到654个益母草潜在靶点。
序号 化合物 来源数据库 1 没食子酸 BATMAN-TCM 2 水苏糖 BATMAN-TCM 3 芦丁 BATMAN-TCM 4 月桂酸 BATMAN-TCM 5 水苏碱 BATMAN-TCM 6 益母草素 BATMAN-TCM 7 西班牙夏罗草酮 BATMAN-TCM 8 鸟嘌呤 BATMAN-TCM 9 益母草碱 BATMAN-TCM 10 4-胍基丁醇 BATMAN-TCM 11 鼬瓣花二萜 TCMSP 12 ZINC04073977 TCMSP 13 前益母草二萜 TCMSP 14 异前益母草二萜 TCMSP 15 槲皮苷 TCMSP 16 花生四烯酸 TCMSP 17 异鼠李素 TCMSP 18 山奈酚 TCMSP 19 葫芦巴碱 文献 -
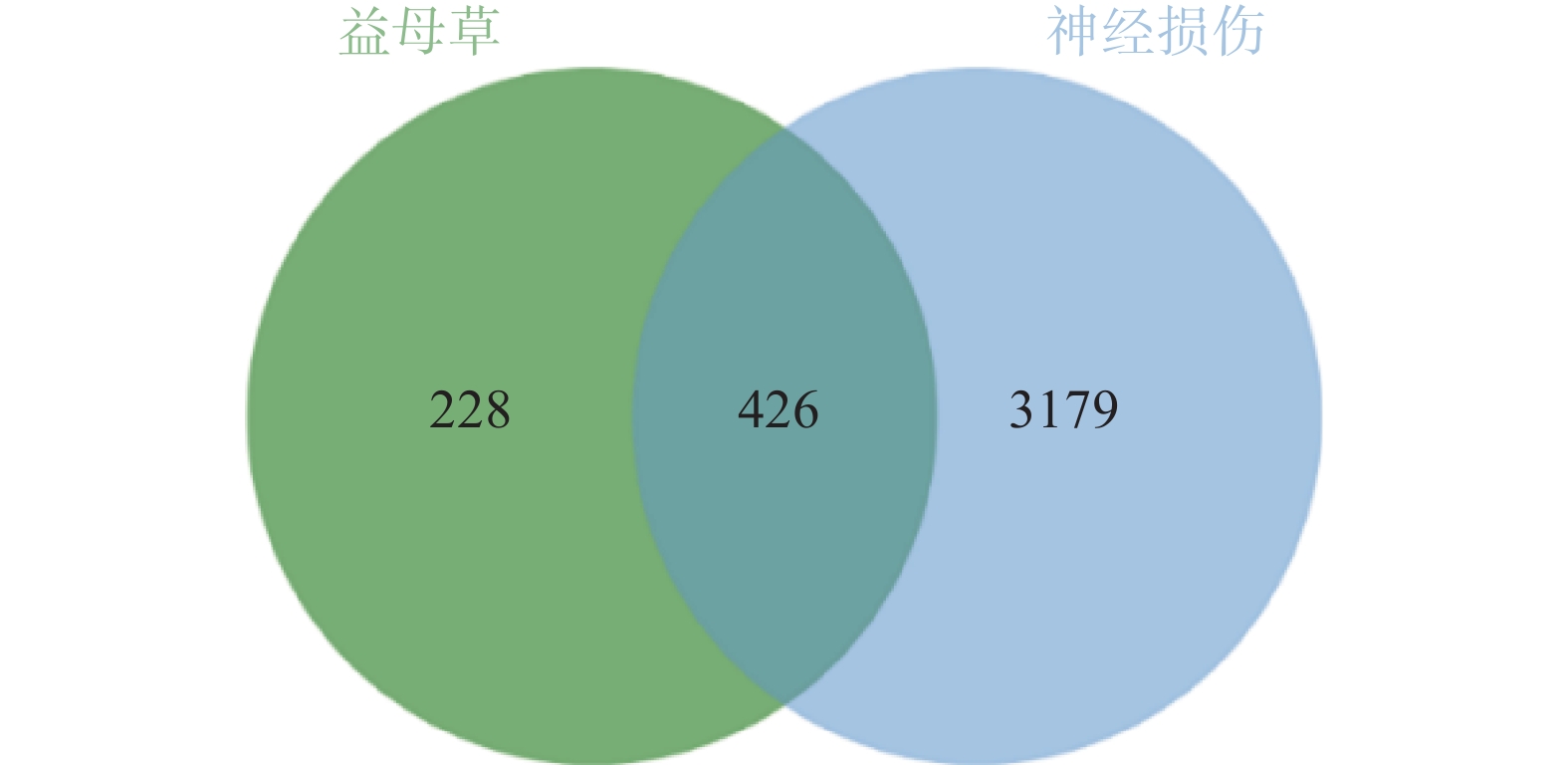
通过GeneCards、DisGenet和OMIM数据库以疾病名称为“cerebral ischemia”和“traumatic brain injury”分别进行检索,在DisGenet数据库中没有检测到TBI靶点,删除重复值后,得到神经损伤的靶点3605个,将疾病相关的靶点与益母草靶点进行Venn交集分析,筛选得到益母草治疗神经损伤的潜在靶点426个,并获得药物-疾病共同靶点基因韦恩图(图1)。
-
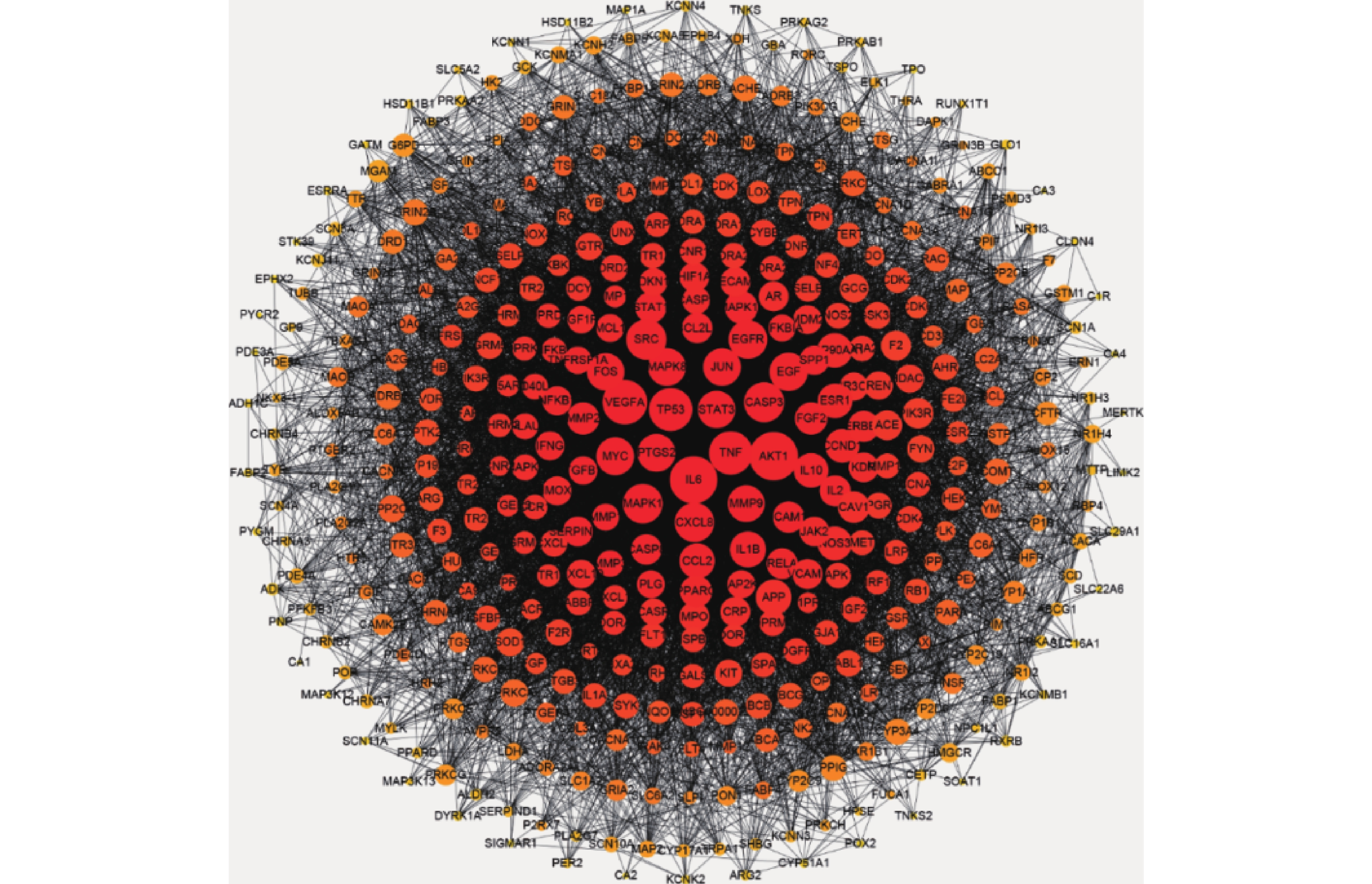
将益母草治疗神经损伤的426个潜在靶点,导入STRING数据库,将相互作用靶点的结果导入Cytoscape 3.6.0进行可视化分析,得到由331个节点、6955条边共同组成的网络(图2),同时得到网络中关键靶点的度值(表2)。如图2所示,与神经损伤相关度较高的靶点(度值≥139)为丝氨酸/苏氨酸蛋白激酶1(AKT1)、白细胞介素6(IL-6)受体、血管内皮生长因子A(VEGFA)、半胱氨酸蛋白酶3(CASP3)、肿瘤蛋白P53(TP53)、基质金属蛋白酶-9 (MMP9)。度值大的靶点提示在网络调控中起关键作用,这些度值大的靶点很可能是益母草治疗神经损伤的关键靶点。
基因 度值 靶点名称 数据库中代码 AKT1 225 丝氨酸/苏氨酸蛋白激酶 P31749 IL6 217 白介素6 P05231 VEGFA 196 血管内皮生长因子A P15692 TNF 187 肿瘤生长因子 P01375 TP53 186 细胞肿瘤抗原P53 P04637 SRC 165 原癌基因酪氨酸受体激酶 P12931 CASP3 163 胱天蛋白酶-3 P42574 MAPK1 160 丝裂原活化蛋白激酶1 P28482 CXCL8 157 白介素8 P10145 EGFR 153 表皮生长因子受体 P00533 EGF 150 前表皮生长因子 P01133 PTGS2 146 牛前列腺素G/H合成酶2 P35354 MAPK8 146 丝裂原活化蛋白激酶8 P45983 MYC 146 原癌基因蛋白Myc P01106 JUN 145 转录因子AP-1 P05412 STAT3 143 信号传导及转录激活子3 P40763 FOS 143 原癌基因c-Fos P01100 MMP9 139 基质金属蛋白酶9 P14780 IL-1β 133 白介素1β P01584 -
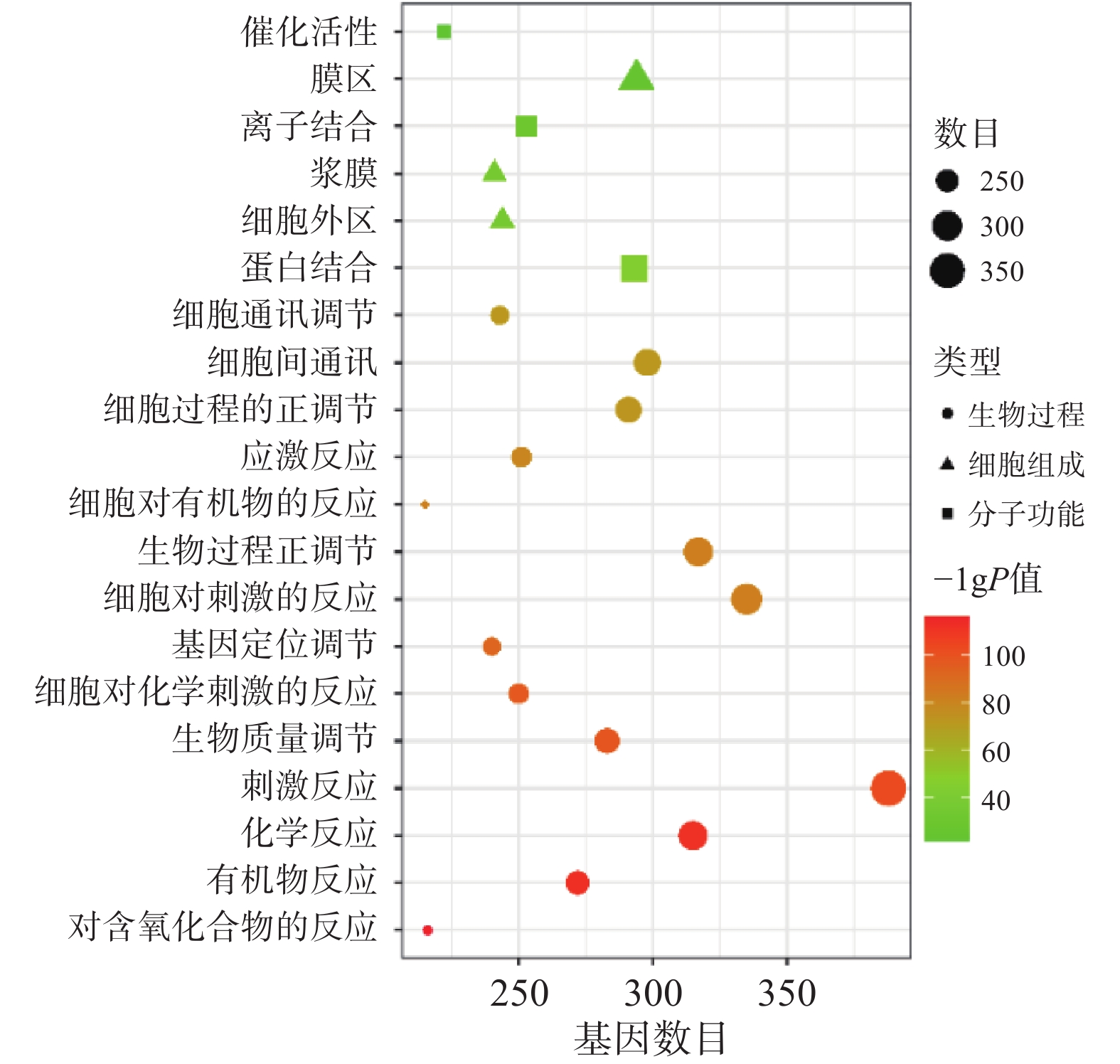
将“2.2” 项下获取的426个潜在的治疗神经损伤的靶点通过STRING进行生物过程(BP)、细胞组分(CC)和分子功能(MF)分析,以 P<0.01为条件,筛选靠前的GO富集分析,如图3所示。图中纵坐标表示富集条目,横坐标表示基因计数,颜色深浅代表-log10(p)值大小。其中 GO-BP 主要为应激反应、生物调节和细胞通讯等;GO-CC主要为细胞膜等;GO-MF主要为蛋白质结合、离子结合和催化还原活性等。
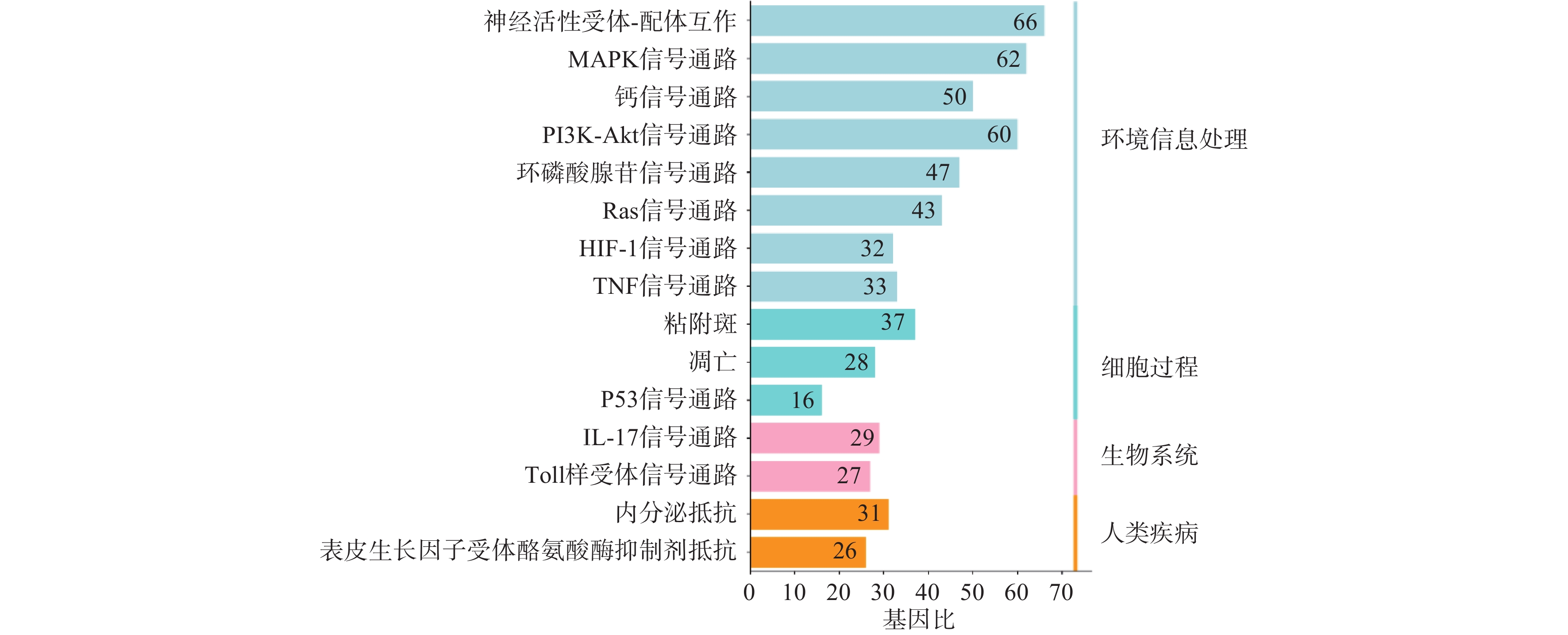
利用STRING数据库对益母草治疗神经损伤的潜在的426个靶点进行富集分析,筛选出显著的前16条信号通路(P<0.01),主要涉及的信号通路为MAPK、Toll样受体、PI3K-Akt、肿瘤坏死因子、IL-17和凋亡等信号通路(图4)。
-
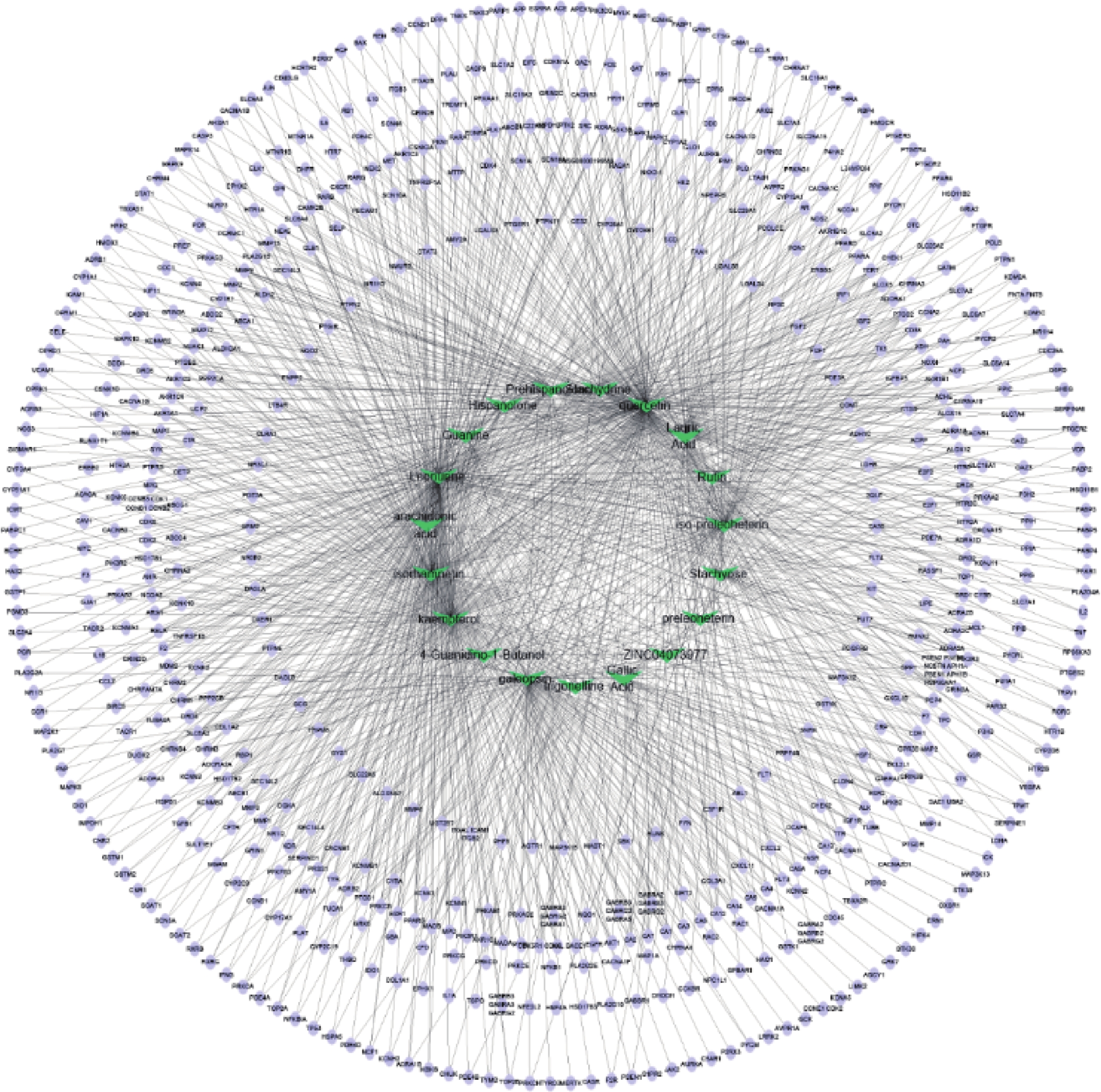
通过Cytoscape3.6.0软件得到药物“活性成分-靶点”的网络(图5)。网络中化合物19个,靶点426个。在图5中,箭头节点代表益母草中化合物,椭圆形节点代表靶点,结果可以明显看出益母草中度值相对较高的化合物有槲皮素、益母草碱、山奈酚、异鼠李素、水苏碱、葫芦巴碱等,这些化合物可能是益母草治疗神经损伤的关键化合物。
-
本研究采用网络药理学方法,借助相关数据库以及各种绘图软件对益母草治疗神经损伤的物质基础和作用机制进行研究。共筛选出益母草有效活性成分19个,对应活性成分靶点654个。其中,益母草与神经损伤的共同基因有426个,“药物活性成分-共同靶点”网络与PPI网络结合分析,结果发现,益母草治疗神经损伤的关键活性成分有槲皮素、益母草碱、山奈酚、异鼠李素、水苏碱、葫芦巴碱等,其中槲皮素对脑缺血的作用最为突出。槲皮素通过发挥抗氧化[12]、抗炎和抗凋亡作用[13]对脑缺血的病理学改变产生了积极的治疗作用。益母草碱通过抗氧化、抗凋亡、保护线粒体和激活Nrf-2/HO-1信号通路发挥血脑屏障保护作用[14]。山萘酚具有抗氧化、抗炎、抗癌和预防心血管疾病等多种药理活性[15]。异鼠李素具有保护心脑血管、抗肿瘤、抗炎、抗氧化、保护器官、预防肥胖等作用[16]。水苏碱通过多种分子机制(包括TGF-β、ers介导的细胞凋亡、MMPs/TIMPs、NF-κB和JAK/STAT)抑制细胞外基质(ECM)沉积、降低炎症和氧化应激,以及血管生成保护血管等发挥神经保护作用[17]。葫芦巴碱具有抗凋亡、抗炎、抗氧化、神经保护等多种药理作用,具有改善认知能力的潜力。
根据益母草靶蛋白互作网络图分析可知,益母草治疗神经损伤的核心靶点主要为:AKT1,IL-6,VEGFA,CASP3, TP53,MMP-9。AKT1能够通过丝氨酸和/或苏氨酸磷酸化介导调节细胞代谢、增殖、细胞存活、胰岛素信号传导、生长和血管生成过程。据报道[18]阿托伐他汀通过抑制JNK3/cJun/caspase-3,增强Akt-nNOS信号通路,抑制脑缺血大鼠脑内细胞凋亡,对脑缺血再灌注有保护作用。IL-6可调节多种细胞的生长与分化,具有调节免疫应答、急性期反应及造血功能,并在机体的抗感染免疫反应中起重要作用。IL-6作为促炎细胞因子在脑缺血中的作用可能是通过NF-κB通路来实现的[19]。据报道,LncRNA MEG8通过miR-130a-5p/VEGFA信号靶向减轻缺血性中风后的脑缺血[20],通过靶向VEGFA,下调microRNA-195促进血管生成[21]。Caspase-3(CASP3)是细胞凋亡途径中最关键的酶类之一,与癌症的发生、衰老、心脑血管疾病的发生等有着重要联系。Nahid等[22]研究发现通过降低Bax/Bcl-2比值和caspase-3活化,可减轻脑缺血后海马CA1神经元损伤,改善脑缺血损伤引起的功能和记忆丧失。TP53是神经元凋亡的主要调节因子,任何降低TP53稳定性及其向线粒体迁移的方法都可以减轻缺血性脑区的神经元损失[23]。MMP-9是一种明胶酶,大脑中许多细胞都能分泌MMP-9。MMP-9降解细胞外基质成分,从而引发中风,Zinnhardt等[24]研究发现脑缺血的发生会促进基质金属蛋白酶(MMPs)的产生,尤其是MMP-9,另外MMP-9的激活又可引起血脑屏障受损。
GO功能富集分析发现,益母草治疗神经损伤的基因功能主要体现在生物调节、氧化应激反应、细胞通讯等生物学过程以及蛋白质结合、离子结合和催化还原等。KEGG信号通路富集分析显示,益母草治疗神经损伤所涉及的TNF信号通路、MAPK信号通路、TP53信号通路、PI3K-Akt信号通路的P值较小,被显著富集。TNF具有促进细胞生长、分化、凋亡及诱发炎症等生物学效应。TNF-α可以激活JNK,Caspase蛋白酶和转录因子NF-kB这三条信号通路,实现其免疫调节和细胞凋亡的生物学功能,从而对脑缺血产生影响。级联p38-MAPK的转导通路位于中枢神经系统,在缺血、缺氧等条件刺激下可被激活。可通过p38 MAPK和c-Jun抑制炎症反应,对损伤后的神经有保护作用[25]。Yao等[26]发现通过抑制MAPK信号通路的激活,恢复神经功能,减轻血脑屏障通透性破坏,对脑缺血产生保护作用。缺血神经元释放的内源性配体激活TLR信号通路,导致大量炎症细胞因子TNF-α、IL-1β、iNOS的产生,从而引起脑缺血后继发性炎症损伤。TLRs介导的缺血耐受可作为预防和治疗脑缺血的重要靶点[27]。TP53是一个肿瘤抑制蛋白,调节各种各样基因的表达,包括细胞凋亡等,此外TP53可不依赖其活性,仅作为一个转录因子来引发凋亡通路。抑制NF-κB及下游TP53可显著减轻神经元自噬和凋亡,具有显著的神经保护作用。NF-κB、TP53及其介导的自噬和凋亡在脑缺血再灌注损伤恶化中也起关键作用[28]。PI3K/AKT信号通路是一条与增殖,分化和凋亡相关的信号通路[29]。
综上所述,本研究应用网络药理学的方法预测了益母草治疗神经损伤的主要活性成分和潜在分子机制,但由于所使用平台的数据收录,更新相对滞后,中药活性成分筛选条件口服生物利用度与类药性并不是唯一的标准,因而,研究预测的结果有其局限性。需要在今后的实验研究中进一步阐明和验证益母草中活性成分的作用靶点,从而完善其治疗神经损伤的有效化学成分及作用机制。
Potential molecular mechanism of motherwort in the treatment of nerve injury based on network pharmacology
doi: 10.12206/j.issn.1006-0111.202105106
- Received Date: 2021-05-22
- Rev Recd Date: 2021-10-26
- Available Online: 2022-05-25
- Publish Date: 2022-03-25
-
Key words:
- motherwort /
- network pharmacology /
- nerve injury
Abstract:
| Citation: | ZHANG Qian, GUO Yuchen, DENG Shanshan, ZHANG Yuefan, LI Tiejun. Potential molecular mechanism of motherwort in the treatment of nerve injury based on network pharmacology[J]. Journal of Pharmaceutical Practice and Service, 2022, 40(2): 113-119. doi: 10.12206/j.issn.1006-0111.202105106 |









 DownLoad:
DownLoad: