-
阿霉素(adriamycin,ADR)是蒽环类DNA拓扑异构酶Ⅱ抑制剂,疗效确切,抗肿瘤谱广,临床上常用于治疗血液系统肿瘤和胃癌、肝癌等多种实体瘤。但阿霉素也有多种严重的不良反应,其心脏毒性常导致患者充血性心力衰竭,故临床应用受到很大的限制。在使用阿霉素治疗肿瘤的同时,给予具有心脏功能保护作用的药物对抗其副作用是一种有效的办法[1]。因此,寻找抗阿霉素心脏毒性损伤的药物已成为近年来的研究热点。参附汤出自明代医家薛己《正体类药》,由人参和炮附子按3∶2配伍组成,具有益气回阳固脱的功效,是传统中医治疗慢性心力衰竭的经典名方。参附汤的药理作用机制尚不明确,阿霉素心脏毒性的发生机制也还在探索中。研究表明参附汤可通过调节microRNAs的表达,抑制线粒体途径的细胞凋亡,改善血流动力学相关指标,抑制心肌细胞自噬等途径发挥心脏保护作用[2-4]。这些研究对参附汤的药理学作用机制进行了有益的探索,但仍不够深入,目前,鲜见从代谢组学角度对参附汤的药理作用机制进行整体研究的相关报道。
代谢组学是一种快速发展的系统生物学方法,能够对暴露于致病因素或药物治疗的复杂生物系统的海量代谢产物提供整体的代谢状态分析,正好契合中医“多成分、多靶点、多途径”整合调节的作用特点,是研究中药的药效成分和药理作用机制的强有力手段[5-6]。因此,本研究采用GC-MS血清代谢组学技术,检测经参附汤治疗的阿霉素心肌病小鼠血清中代谢物的变化,通过多变量统计分析寻找潜在生物标志物,综合评价参附汤抗阿霉素心肌病的作用,以期从整体、多靶点的层面,深入阐释参附汤抗阿霉素心肌病的作用机制。
-
Thermo-Finnigan Trace DSQ气相色谱-质谱联用仪(Thermo Electron Corporation);Vevo-3100LT高分辨超声显像系统(加拿大Visual Sonics公司)。
-
乳酸脱氢酶(LDH)、肌酸磷化酶-同功酶MB试剂盒(CK-MB)(南京建成生物科技有限公司);三甲基氯硅烷(TMCS)、甲氧胺盐酸盐、N-甲基-N-三甲基硅烷基三氟乙酰胺(MSTFA)、甲醇、吡啶、正庚烷、柠檬酸(美国Sigma-Aldrich公司);酪氨酸、苯丙氨酸、丙氨酸、油酸、硬脂酸、乳酸、琥珀酸、缬氨酸、甘油-3-磷酸、苹果酸、花生四烯酸(上海阿拉丁试剂有限公司);炮附子(产地:四川)、人参(产地:吉林)购自西安市北京同仁堂药房。
-
BALB/c雄性小鼠24只,体重(20±3)g,空军军医大学实验动物中心提供。小鼠饲养条件:恒温(25±1)℃,12 h/12 h昼夜的房间,自由进食标准饲料和纯净水。
-
称取人参60 g,炮附子40 g,采用6倍体积的沸水法提取2次,每次提取2 h,合并2次提取液,减压浓缩至浓度为0.45 g/ml(相当于含人参0.3 g和制附子0.2 g)的参附汤溶液。
-
经过1周的适应性饲养后,24只BALB/c小鼠随机分为3组(正常组、阿霉素模型组、参附汤治疗组),每组8只。模型组:在第1、3、5、7、9、11 d分别腹腔注射阿霉素2、2、3、3、3、3 mg/(kg·d),累计剂量16 mg/kg。参附汤治疗组:在阿霉素造模前3 d,每天灌胃给予参附汤原液1 g/(kg·d),造模的同时继续给药,造模后再连续给药12 d。正常组:腹腔注射等量的生理盐水。
-
各组最后一次给药后72 h,将小鼠用1.5%~2%的异氟烷麻醉,然后行超声心动图检查,评估左心室收缩功能,射血分数(EF)和缩短分数(FS)通过标准公式计算而得。
-
小鼠眼眶取血,血样在室温下放置45 min,4℃下3 000 r/min离心15 min,所得血清一部分用于LDH、CK-MB酶分析,另一部分置于−80 ℃冰箱冻存,用于代谢组学分析。代谢组学质量控制样品(QCs)取自合并等量不同小鼠的血清。
-
将冻存的血清于室温下融化,取100 μl血清,加入300 μl甲醇,涡旋1 min,在冰水浴中孵育10 min,4℃下14 000 r/min离心15min,放置于4℃冰箱中保存。GC-MS分析前,取上清液转移到玻璃瓶,室温下氮气吹干。衍生化:在血清干燥物中加入15 mg/ml的甲氧胺吡啶溶液50 μl,涡旋5 min,70℃ 烘箱放置1 h。加入50 μl MSTFA(含有1% TMCS),涡旋5 min,室温放置1 h;加入150 μl正庚烷,涡旋30 s,4 000 r/min离心10 min,取上清液150 μl至样品瓶。
-
GC-MS分析采用TR-5MS毛细管柱(30 m×250 μm,0.25 μm),进样量1 μl,分流比为10:1,载气为氦气,柱流速为1 ml/min,进样口温度为260 ℃;接口温度为260 ℃;离子源温度为200 ℃;四级杆温度150℃,EI离子源70 eV;采用全扫描模式,m/z:60~600;程序升温条件:70℃保持3 min,以4℃/min升至220 ℃,然后以12℃/min升至310℃后,保持10 min。样品分为8个小组进样,每小组的3个样品分别取自正常组、阿霉素组、参附汤治疗组,每个小组内采用随机方式进样,每个小组之间进样一次QCs,用于监测分析系统的稳定性。
-
原始数据经ThermoXconvert软件转为NetCFD数据格式,进一步通过XCMS软件包(参数设定fwhm=1,bw=2,snthersh=5,其余默认值)进行峰校正和峰积分[7],处理获得的矩阵导入Excel,对数据进行归一化处理,采用SIMCA-P 14.1软件进一步进行中心化和标准化处理,然后进行主成分分析和正交偏最小二乘法判别分析。通过S-plot载荷图寻找生物标志物,对差异代谢物做进一步双侧t检验分析(阿霉素组与正常组)和方差分析(P<0.05)。代谢物的鉴定通过标准质谱数据库(NIST数据库)检索匹配,并采用对照品对部分代谢物进行确认。
-
潜在生物标志物的代谢通路分析采用基于网页来源的Metaboanalyst(http://www.metaboanalyst.ca)软件进行分析[8]。
-
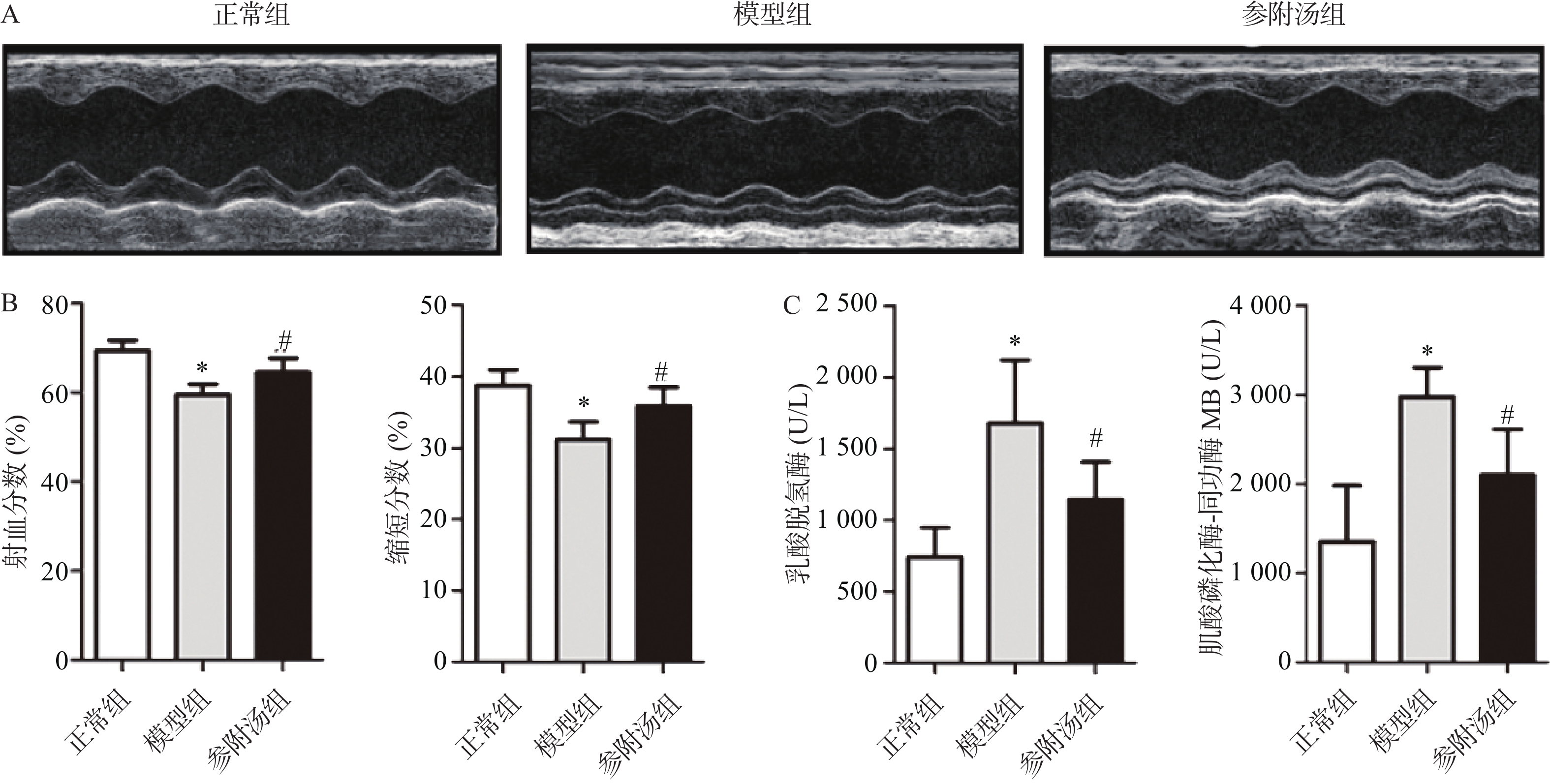
血清中LDH、CK-MB的测定结果和超声心动图如图1所示,与正常组相比,模型组血清LDH、CK-MB值明显升高,EF、FS值明显下降,表明造模成功,模型组小鼠心脏功能受损。经参附汤治疗后,与模型组相比,参附汤治疗组LDH、MB值明显降低(P<0.05),EF、FS值明显升高(P<0.05)。血清酶指标和超声心动图结果表明参附汤能够改善阿霉素引起的心功能损伤。
-
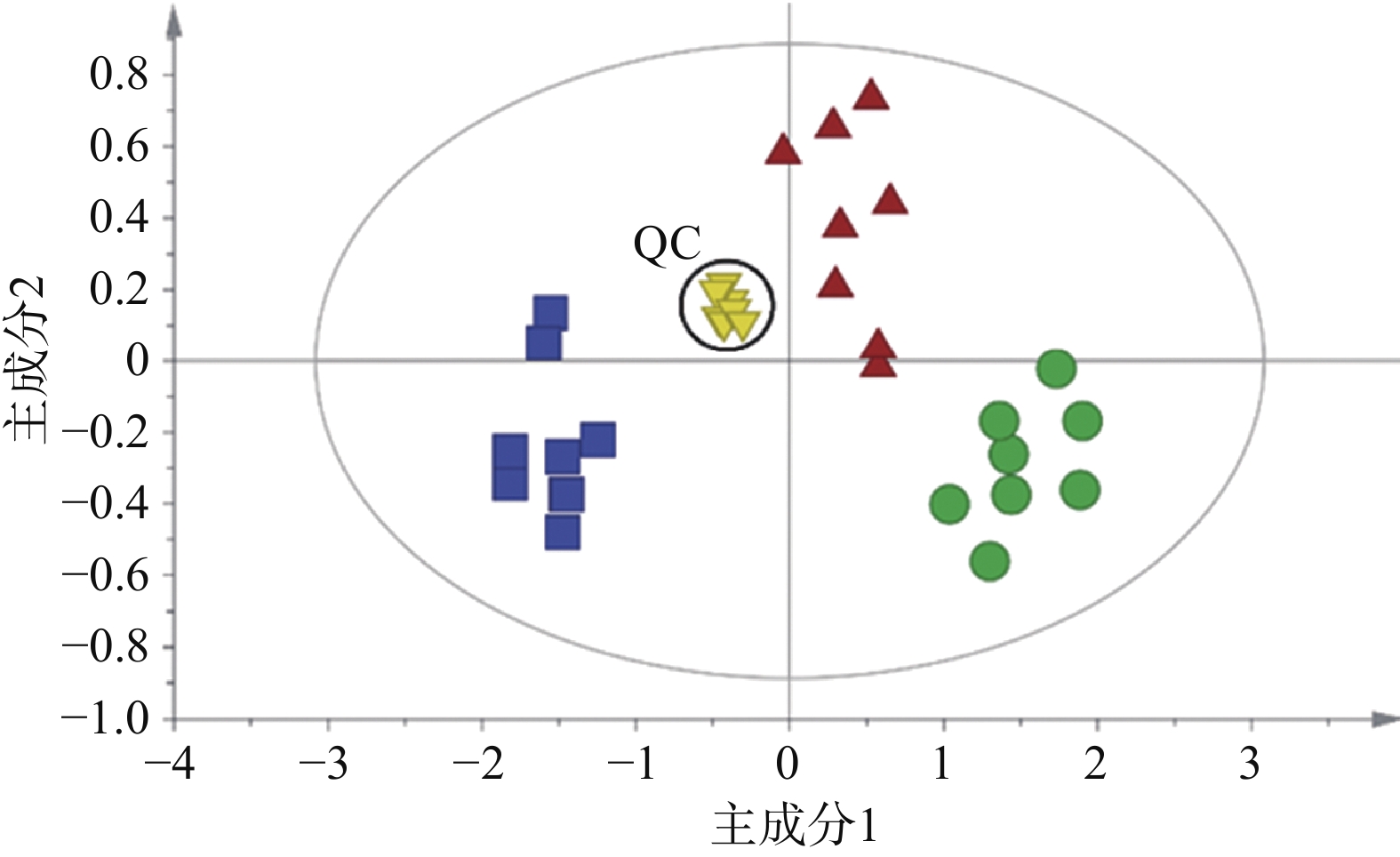
采用QCs评价GC-MS系统的稳定性。整个分析过程一共进样9次QCs,不同组样品与QCs通过XCMS校正、积分、提取特征离子峰,一共得到352个离子峰,设定峰面积相对标准偏差小于30%提取变量,结果得到319个变量的数据集,占总变量数的90.6%。可见本研究建立的GC-MS分析系统稳定性良好。无监督的主成分分析结果如图2所示,可见9个QC紧密聚集在一起,这也进一步证实了分析系统具有良好的稳定性。
-
为研究小鼠心脏功能受损前后血清代谢物的差异与变化,并评估参附汤对小鼠受损心脏功能的保护作用,将实验获取的所有数据进行主成分分析。主成分分析能够将不同组别的样本区分开来,并且指示特征离子。将正常组和模型组的GC-MS数据进行正交偏最小二乘法判别分析,得出反映两组间离散程度的得分图(图3A)和寻找相关生物标志物的S-plot载荷图(图3B)。从得分图可以看出,正常组与模型组能实现明显分离,表明模型组小鼠心脏功能受损后,内源性物质代谢发生紊乱,血清代谢谱出现明显变化。S-plot载荷图能指引找到对2组分离贡献较大的代谢物,离原点越远贡献越大。利用S-plot载荷图,再结合峰面积相对强度的双侧t检验分析,一共鉴定了13种阿霉素心脏毒性潜在的生物标志物,如图4所示。
在这13种潜在的生物标志物中,最常见的是氨基酸代谢失衡。模型组丙氨酸、苯丙氨酸水平升高,而异亮氨酸、缬氨酸水平降低。氨基酸是能量代谢的重要前体,能增加ATP的合成,另外苯丙氨酸是合成儿茶酚胺的重要原料,在应激状态下儿茶酚胺合成增多。其次是长链脂肪酸代谢受阻,模型组硬脂酸、油酸水平显著升高,而它们是正常心肌细胞能量代谢的主要原料。从三组的主成分分析得分图(图2)可以发现,参附汤治疗组与模型组、正常组之间均能分开,并且更加靠近正常组。此外,经参附汤治疗后,所鉴定的13种阿霉素心脏毒性潜在的生物标志物中的11种明显回调,如表1所示,这些结果与小鼠血清生化指标及超声心动图的改善相一致,表明参附汤能通过整体逆转小鼠血清代谢谱,从而发挥抗阿霉素诱导的心脏毒性的作用。
序号 保留时间(min) 代谢物 基峰 m/z MS 碎片 峰相对强度 正常组 模型组 参附汤组 1 8.89 乳酸 73 191, 147, 117 (2.04±0.28)×10−1 (4.36±0.21)×10−1 ↑* (2.79±0.54)×10−1 ↓# 2 10.14 丙氨酸 116.1 190, 147, 73 (7.95±1.47)×10−3 (1.55±0.22)×10−2 ↑* (1.07±0.28)×10−2 ↓# 3 12.38 异亮氨酸 86.1 188, 147, 117, 73 (1.20±0.12)×10−3 (6.62±0.78)×10−4 ↓* (9.69±1.11)×10−4 ↑# 4 13.89 缬氨酸 144.1 218, 73 (2.02±0.41)×10−2 (1.03±0.11)×10−2 ↓* (1.71±0.39)×10−2 ↑# 5 17.04 琥珀酸 247.1 147, 75, 73 (6.45±0.90)×10−5 (1.11±0.23)×10−4 ↑* (7.02±1.62)×10−5 ↓# 6 22.87 苹果酸 73 233, 189, 147 (8.88±1.19)×10−4 (1.67±0.20)×10−3 ↑* (1.04±0.26)×10−3 ↓# 7 24.03 苯丙氨酸 120.1 146, 91, 73 (8.79±1.07)×10−4 (1.57±0.20)×10−3 ↑* (1.15±0.15)×10−3 ↓# 8 30.84 甘油-3-磷酸 299.1 357, 147, 73 (7.95±0.83)×10−5 (1.25±0.18)×10−4 ↑* (1.08±0.14)×10−4 ↓ 9 32.25 柠檬酸 273.11 147, 73 (5.00±0.64)×10−4 (3.04±0.53)×10−4 ↓* (4.33±0.56)×10−4 ↑# 10 33.36 酪氨酸 179.1 208, 73 (1.70±0.30)×10−3 (1.04±0.17)×10−3 ↓* (1.25±0.31)×10−3 ↑ 11 40.85 油酸 73.01 339, 129, 117 (2.22±0.55)×10−3 (3.61±0.63)×10−3 ↑* (2.51±0.51)×10−3 ↓# 12 41.34 硬脂酸 117 341, 145, 129 (1.76±0.63)×10−3 (3.00±0.40)×10−3 ↑* (2.11±0.45)×10−3 ↓# 13 43.10 花生四烯酸 93.1 133, 117, 91 (1.57±0.11)×10−4 (2.48±0.33)×10−4 ↑* (1.72±0.28)×10−4 ↓# 注:*P<0.05,与正常组比较;#P<0.05,与模型组比较 -
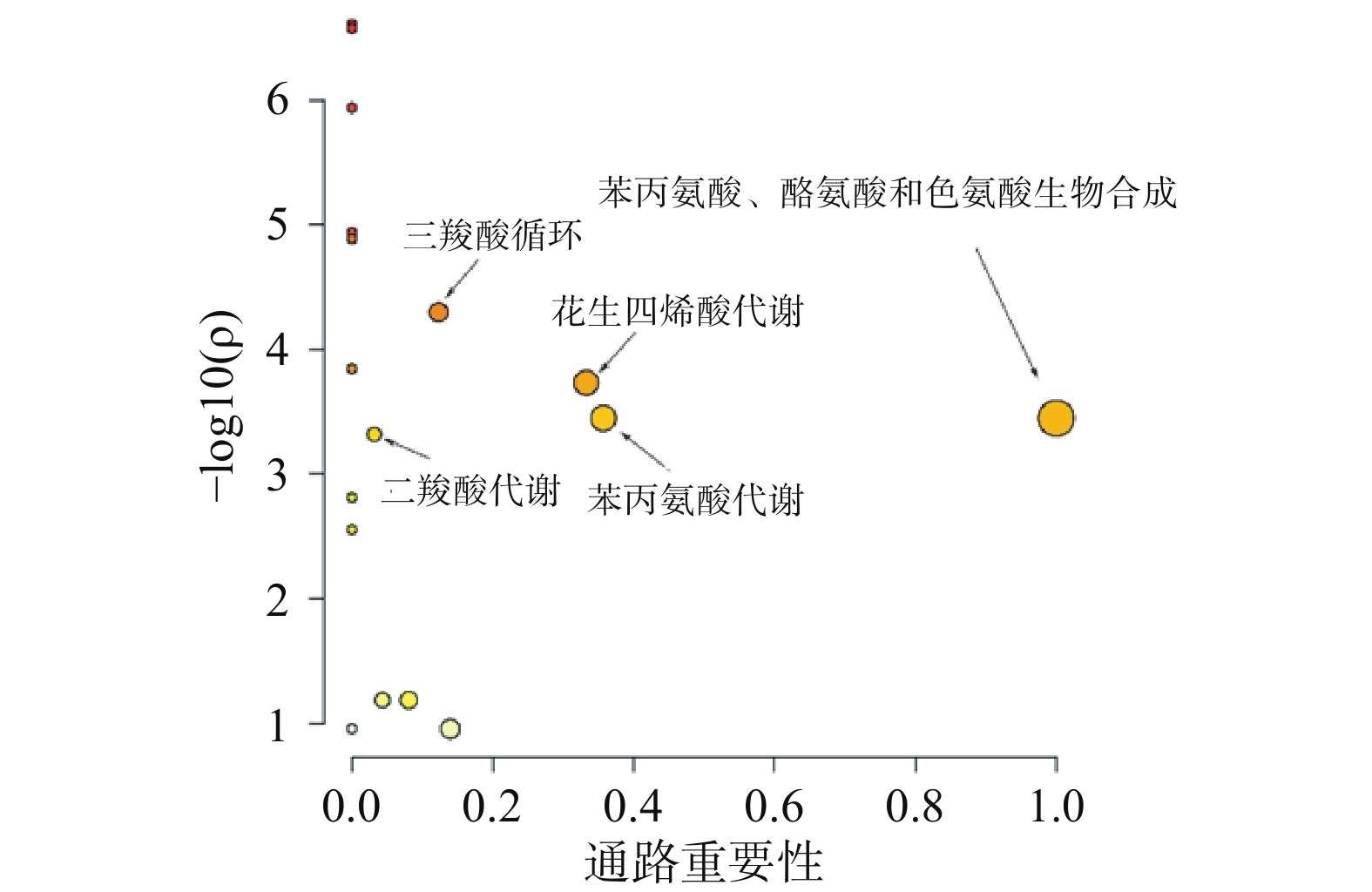
采用Metaboanalyst软件对逆转代谢标志物在参附汤治疗组与阿霉素组的相对峰面积进行代谢通路分析,以代谢通路重要性>0.1和统计学分析P<0.05为靶向通路筛选界值,结果如图5所示。表明苯丙氨酸、酪氨酸和色氨酸的合成、花生四烯酸代谢、苯丙氨酸代谢、三羧酸循环和二羧酸代谢通路是参附汤主要的靶向代谢通路。其中苯丙氨酸、酪氨酸和色氨酸是蛋白质代谢的重要成分,花生四烯酸代谢是炎症调控代谢途经,三羧酸循环、二羧酸代谢是心肌能量代谢的重要途经。表明参附汤可以通过改善心肌蛋白质代谢、能量代谢和减轻炎症效应,从而发挥抗阿霉素心脏毒性的作用。
-
本研究建立了一种基于GC-MS的代谢组学研究方法,用于探索阿霉素对小鼠心脏毒性以及参附汤抗阿霉素心脏毒性的作用机制。单变量和多变量统计学分析结果提示正常组、模型组和参附汤治疗组的代谢谱明显不同,一共鉴定出13种潜在阿霉素心脏毒性血清生物标志物,以这些生物标志物为潜在的药物靶标,参附汤能够明显逆转其中11种代谢物,表明参附汤对阿霉素所致的心功能损伤有治疗作用。多变量统计学分析代谢通路表明,苯丙氨酸、酪氨酸和色氨酸的合成、花生四烯酸代谢、苯丙氨酸代谢、三羧酸循环和二羧酸代谢是参附汤主要的靶向代谢通路。
Serum metabolomic study of Shenfu decoction on adriamycin-induced cardiomyopathy in mice
doi: 10.12206/j.issn.1006-0111.202105116
- Received Date: 2021-05-25
- Rev Recd Date: 2021-08-21
- Available Online: 2022-05-25
- Publish Date: 2022-03-25
-
Key words:
- adriamycin /
- cardiomyopathy /
- Shenfu decoction /
- GC-MS /
- metabolomics
Abstract:
| Citation: | QIN Ye, DIN Xin, ZHANG Ya. Serum metabolomic study of Shenfu decoction on adriamycin-induced cardiomyopathy in mice[J]. Journal of Pharmaceutical Practice and Service, 2022, 40(2): 108-112. doi: 10.12206/j.issn.1006-0111.202105116 |










 DownLoad:
DownLoad: