-
复方金钱草颗粒由广金钱草、车前草、光石韦以及玉米须四味中药材组成,现执行标准为《中国药典》(一部)2015年版及国家食品药品监督管理总局药品补充批件(2016B00026)。该药具有清热利湿、通淋排石的作用,在临床上用于治疗湿热所致的热淋、石淋,症见尿频、尿急、尿痛、腰痛的患者,以及泌尿系结石、尿路感染见上述证候者[1]。现代药理学研究也显示复方金钱草颗粒具有防治草酸钙结石形成、促进输尿管蠕动和增加尿量、抗炎[2-5]及增加冠脉血流量、保肝利胆[3-5]、免疫调节等作用[5]。复方金钱草颗粒中的化学成分包含黄酮类[5-8]、甾醇类[5,7-9]、生物碱类[5-7]、萜类[5,7,9]、酚酸类[5-6,8]、挥发油[5,7]、多糖类等[6-7]。由于复方金钱草颗粒成分复杂,目前尚无全面的化学成分鉴定工作。本研究致力于建立起一种快速有效的系统分析方法,同时定性地鉴别其多种化合物成分,为其有效成分研究提供依据。
-
分析纯无水乙醇(中国医药集团上海化学试剂公司);质谱纯甲酸、质谱纯乙腈、0.22 µm滤膜(德国Merck集团);复方金钱草颗粒(规格:10g,广西万通制药有限公司)
-
BP121S电子分析天平(德国Sartorius公司);Milli-Q A10超纯水净化系统(美国Millipore公司);KQ-400KDB超声波清洗器(400W, 40KHz;昆山市超声仪器有限公司);X1R-230 V台式离心机(美国Thermo Fisher Scientific公司);Agilent 1290超高效液相色谱仪、Agilent 6538四极杆飞行时间质谱仪(美国安捷伦公司)。
-
采用Agilent 1290超高效液相色谱仪(UHPLC),色谱柱为XBridge BEH C18柱(2.1 mm×100 mm, 2.5 µm ),柱温为40 ℃,进样量为3 µl,流动相由0.1%甲酸水(A)和0.1%甲酸乙腈(B)组成,梯度洗脱,洗脱条件见表1,分析时间为19 min,流速为0.4 ml/min。
时间(t/min) A(%) B(%) 0 98 2 2 98 2 12 40 60 17 2 98 19 2 98 -
采用Agilent 6538四极杆飞行时间质谱仪(Q-TOF/MS),质谱参数如下:电喷雾离子源(正离子模式);质谱扫描范围: 50~1500 m/z;干燥气温度为350 ℃;干燥气流速为11 L/min;雾化气压力为45 psig;毛细管电压为4000 V;碎片电压为120 V;Skimmer电压为60 V;参比离子m/z为121.0509和922.0098。
-
精密称取2.0 g复方金钱草颗粒,置于50 ml具塞三角烧瓶中,加入55 %乙醇提取溶剂25 ml,称定质量并记录,在55 ℃水浴中超声加热回流提取药液10 min,经室温冷却后,再次称定并用提取溶剂补足损失的质量,15000 r/min离心20 min,取上清液并经0.22 µm滤膜过滤,得提取液。
-
复方金钱草颗粒由广金钱草、车前草、光石韦和玉米须等四味中药材组成。通过搜索中国知网、万方数据、PubMed等网站中关于复方金钱草颗粒及其四味中药材的文献,检索上海有机化学研究所的化学专业数据库、TCMID来建立复方金钱草颗粒化学成分数据库,其相关信息包括化合物中英文名称、分子式以及相对分子质量。
-
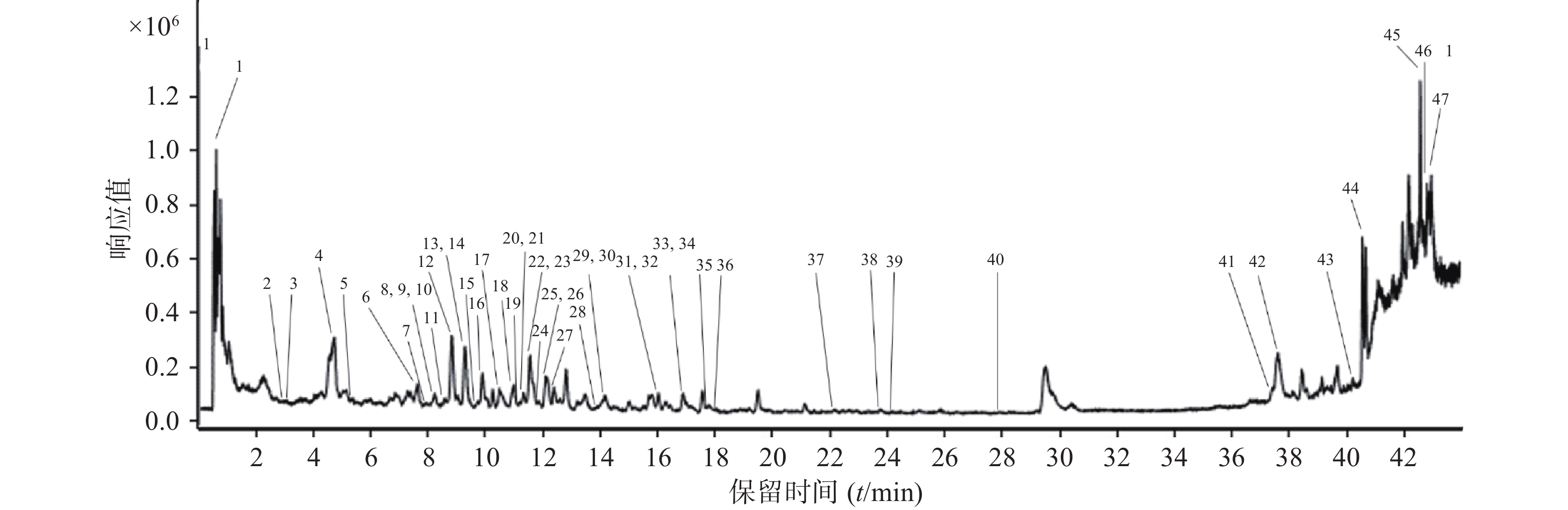
复方金钱草颗粒UHPLC-Q-TOF/MS总离子流图见图1。应用Qualitative Analysis质谱分析软件计算可能的分子组成(误差<8 ppm),结合复方金钱草颗粒化学成分数据库,对复方金钱草颗粒提取溶液所得色谱图上色谱峰进行分析,利用精确分子量匹配和碎片离子归属策略,初步尝试鉴定出51个化学成分,结果如表2所示。
序号 名称 分子式 M+X m/z 质量 误差(ppm) 1 b 水苏糖 C24 H42 O21 (M+H)+ 667.2312 666.2244 3.86 2 b 桂皮酸 C9 H8 O2 (M+H)+ 149.0594 148.052 −3.2 3 abd 原儿茶酸 C7 H6 O4 (M+H)+ 155.0335 154.0261 −3.44 /龙胆酸 4 a 广金钱草内酯 C8 H13 N O3 (M+H)+ 172.0968 171.0894 −0.75 5 bd 咖啡酸 C9 H8 O4 (M+H)+ 181.0493 180.0421 −0.79 6 abcd 绿原酸 C16 H18 O9 (M+H)+ 355.1028 354.0955 1.1 7 b 丁香酸 C9 H10 O5 (M+H)+ 199.0607 198.0531 1.24 8 a 香橙素 C15 H12 O6 (M+H)+ 289.0716 288.0641 2.62 9 ab 黄芩素 C15 H10 O5 (M+H)+ 271.0609 270.0535 2.66 /染料木素 /芹菜素 10 c (R/S)-eriodictyol-8-C-β-D-glucopyranoside C21 H22 O11 (M+H)+ 473.1063 450.118 3.88 11 a 芸香苷 C27 H30 O16 (M+H)+ 611.1623 610.1551 2.79 12 ac 槲皮素 C15 H10 O7 (M+H)+ 303.0505 302.0432 1.78 /3,5,7,4'-tetrahydroxy-coumaronochromone /6-羟基木犀草素 13 b 大车前苷 C17 H24 O10 (M+H)+ 389.1458 388.1385 4.04 14 bd 对-香豆酸 C9 H8 O3 (M+H)+ 165.0547 164.0476 1.45 15 a homoadonivernite C26 H28 O15 (M+H)+ 581.152 580.1445 2.96 /刺苞菊苷 16 a 维采宁-2 C27 H30 O15 (M+H)+ 595.1672 594.1597 2.08 17 b 桃叶珊瑚苷元 C9 H12 O4 (M+H)+ 185.0816 184.0742 3.46 18 a 6-C-glycopyranosyl-8-C-xyloeyl apigenin C26 H28 O13 (M+H)+ 549.1621 548.1552 4.12 /6-C-glycopyranosyl-8-C-glycopyranosyl apigenin 19 ab 阿魏酸 C10 H10 O4 (M+H)+ 195.0654 194.058 0.71 /咖啡酸甲酯 20 b 黑麦草内酯 C11 H16 O3 (M+H)+ 197.117 196.1097 −1.16 21 b 车前黄酮苷 C21 H20 O11 (M+H)+ 449.1089 448.1016 2.34 22 b 木犀草素-7-O-葡萄糖苷 C21 H20 O11 (M+H)+ 449.1089 448.1016 2.34 23 a 异槲皮苷 C21 H20 O12 (M+H)+ 465.1033 464.0965 2.16 /紫花杜鹃素丁 /6-hydroxyl luteolin 7-O-glucoside 24 c 杧果苷 C19 H18 O11 (M+H)+ 423.0933 422.0861 2.84 /异杧果苷 25 a 山柰酚-3-O-芸香糖苷 C27 H30 O15 (M+H)+ 595.1672 594.1597 2.08 26 a 维采宁-1 C26 H28 O14 (M+H)+ 565.1572 564.1498 3.42 /维采宁-3 /异夏佛塔雪轮苷 /夏弗塔雪轮苷 27 abd 对羟基苯甲酸 C7 H6 O3 (M+H)+ 139.039 138.0317 0.31 /水杨酸 /原儿茶醛 28 a 异荭草素 C21 H20 O11 (M+H)+ 449.1089 448.1016 2.34 29 a 牡荆素 C21 H20 O10 (M+H)+ 433.1137 432.1064 1.65 /异牡荆素 /大波斯菊苷 /染料木苷 30 a 三叶豆苷 C21 H20 O11 (M+H)+ 449.1089 448.1016 2.34 31 ab 5, 7-dihydroxy-2', 4'-dimthoxy-isoflavanone-7-O-β-glucopyranoside C23 H26 O11 (M+H)+ 479.1563 478.149 3.11 /[(2R,3S,4S,5R,6R)-6-[2-(3,4-dihydroxyphenyl)ethoxy]-4,5-dihydroxy-2-(hydroxymethyl)oxan-3-yl] (E)-3-(3,4-dihydroxyphenyl)prop-2-enoate /木通苯乙醇苷A /木通苯乙醇苷B /车前草苷 A /车前草苷 B /3,4-Dihydroxyphenethylalcohol-6-O-caffeoyl-β-D-glucoside /去鼠李糖洋丁香酚苷 32 b 车前草苷D C29 H36 O16 (M+H)+ 663.19 640.1989 −2.26 /大车前苷 33 b 异洋丁香酚苷 C29 H36 O15 (M+H)+ 647.195 624.2038 −2.67 /洋丁香酚苷 /Beta-D-Glucopyranoside, 2-(3,4-dihydroxyphenyl)ethyl 3-O-(6-deoxy-alpha-L-mannopyranosyl)-, 6-(3-(3,4-dihydroxyphenyl)-2-propenoate), (E)- 34 b 黄芩苷 C21 H18 O11 (M+H)+ 447.0938 446.0864 3.35 35 b 高车前苷 C22 H22 O11 (M+H)+ 463.1239 462.117 1.7 36 a 5, 7-Dihydroxy-2'-methoxy-3', 4'-methylenedioxy-isoflavanone-7-O-β-glucopyranoside C23 H24 O12 (M+H)+ 493.136 492.1282 2.96 37 abcd 山柰酚 C15 H10 O6 (M+H)+ 287.0558 286.0482 1.45 /木犀草素 /2’-Hydroxygenistein /高山黄芩素 38 a 5, 7-Dihydroxy-2', 3', 4'-trimethoxy-isoflavanone-7-O-β-glucopyranoside C24 H28 O12 (M+H)+ 509.1668 508.1596 2.93 39 b 异角胡麻苷 C31 H40 O15 (M+Na)+ 675.2281 652.2391 3.69 /角胡麻苷 40 a 5, 7-Dihydroxy-2'-methoxy-3', 4'-methylenedioxy-isoflavanone C17 H14 O7 (M+H)+ 331.0824 330.0748 2.45 41 a 5, 7-Dihydroxy-2', 3', 4'-trimethoxy-isoflavanone C18 H18 O7 (M+H)+ 347.112 346.105 −0.6 42 a Homoferreirin C17 H16 O6 (M+H)+ 317.1034 316.0959 3.82 43 a 大豆皂苷 I C48 H78 O18 (M+H)+ 965.5112 942.5214 2.75 44 b 桃叶珊瑚苷 C15 H22 O9 (M+H)+ 347.1352 346.1279 4.44 45 d 亚油酸 C18 H32 O2 (M+H)+ 303.2303 280.2408 2.2 46 d 邻苯二甲酸二丁酯 C16 H22 O4 (M+H)+ 301.1421 278.1531 4.47 47 d Bis[(2R)-2-ethylhexyl] benzene-1, 2-dicarboxylate C24 H38 O4 (M+H)+ 413.2681 390.2798 7.14 注:a. 广金钱草中的成分;b. 车前草中的成分;c. 光石韦中的成分;d. 玉米须中的成分 -
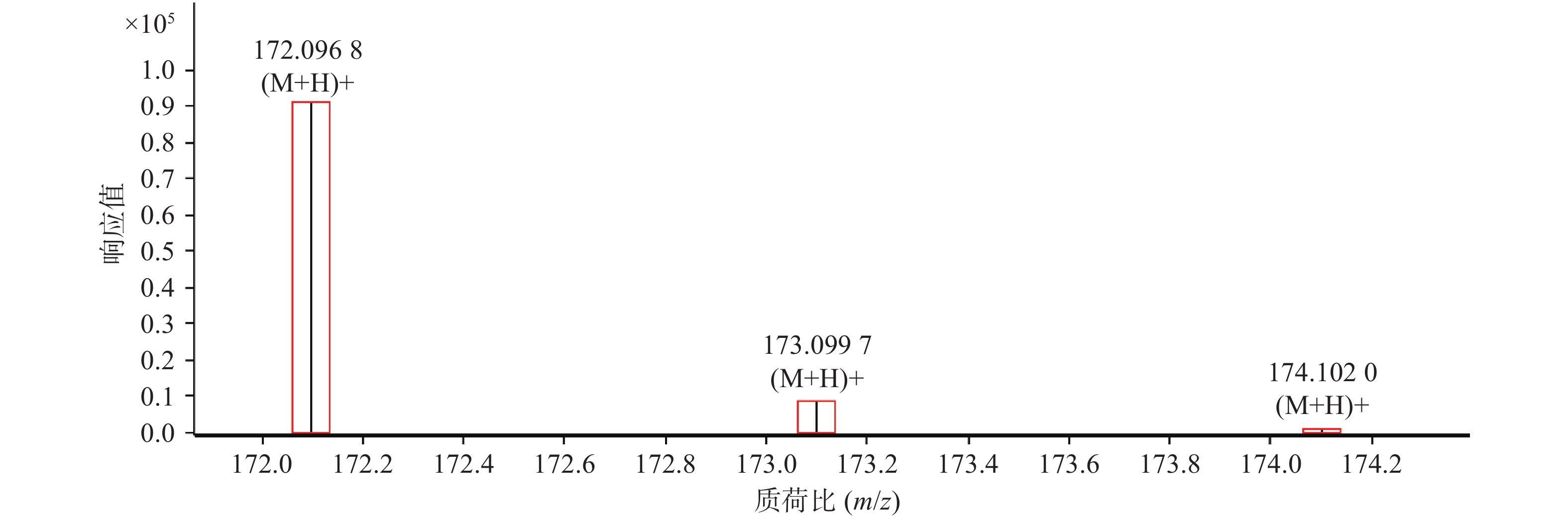
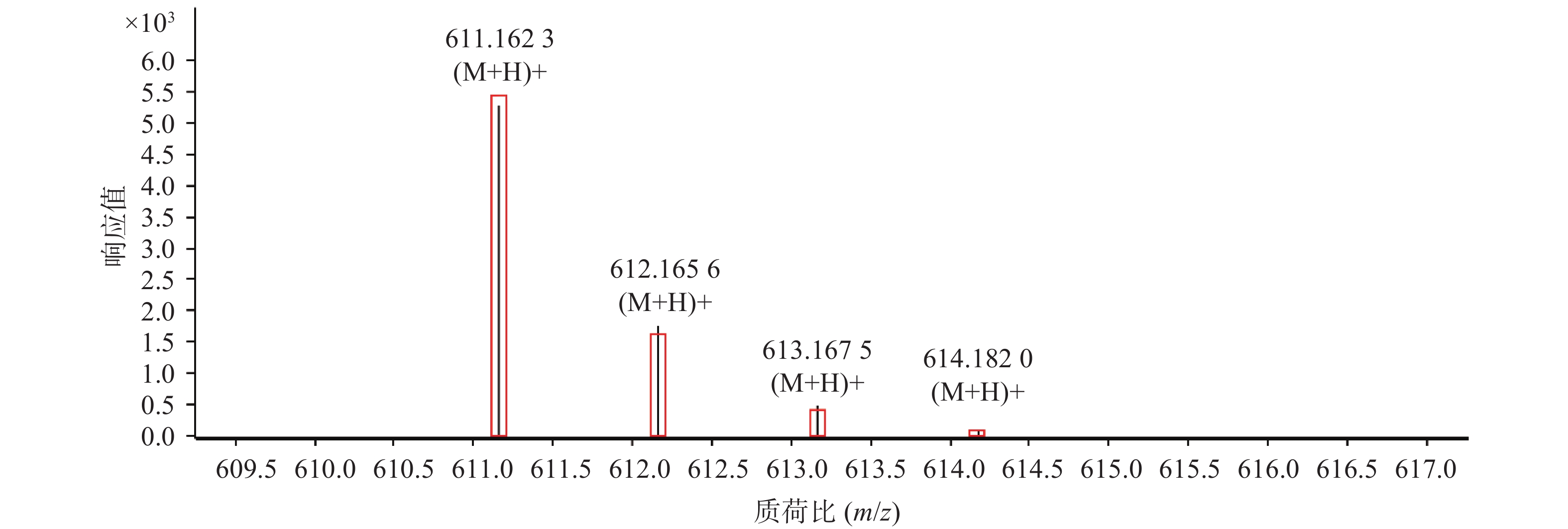
对于以上鉴别分析结果,在数据匹配的基础上,我们还利用峰物质特征的同位素分布进行验证。以4号峰广金钱草内酯和11号峰芸香苷为例,采用Qualitative Analysis软件的“显示预测的同位素分布”功能分别对这两种化合物的同位素峰进行匹配,其结果见图2和图3。图中结果明显可见,该两种离子理论上的同位素峰强度比、出峰位置(由方框表示)与实际测得的(由竖线峰表示)结果吻合良好,4号化合物广金钱草内酯的吻合分数为99.94,11号化合物芸香苷的吻合分数为95.28。以上结果证明鉴别分析结果准确。
-
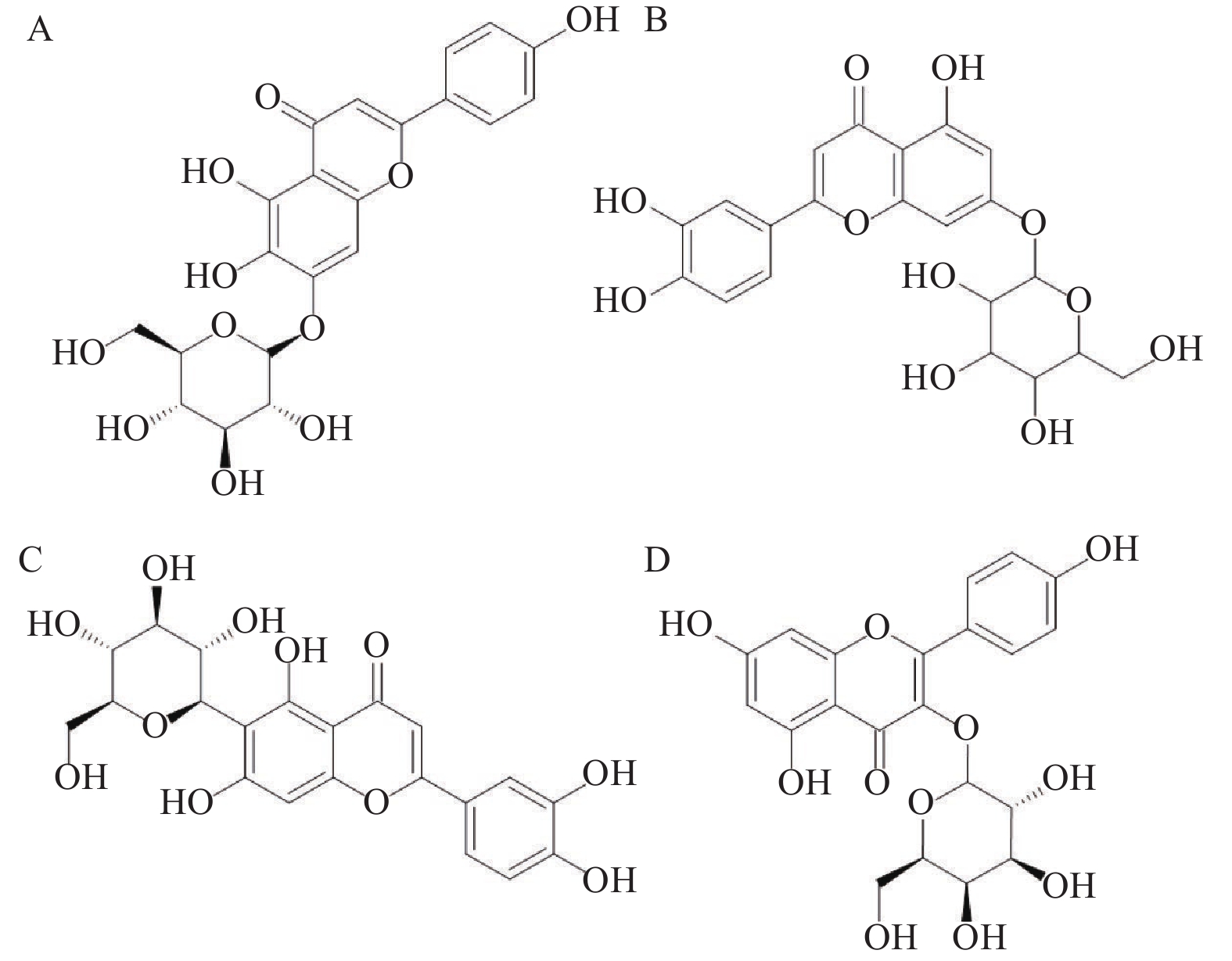
实验中我们发现复方金钱草颗粒中含有多组同分异构体,本文采用ACD/ChemSketch软件计算化合物的油水分配系数logP值判断出峰顺序。以16号峰维采宁-2与25号峰山柰酚-3-O-芸香糖苷为例,在UHPLC-Q-TOF/MS总离子流图中,提取m/z为595.1672的离子,结果见图4。
维采宁-2和山柰酚-3-O-芸香糖苷的结构式如图5所示。采用ACD/ChemSketch软件计算它们的油水分配系数logP值分别为−0.10(±0.94)和1.96(±1.45),表明山柰酚-3-O-芸香糖苷的疏水性更强,在反相色谱柱中的保留时间则越长,确定这两个化合物的出峰顺序维采宁-2在前,山柰酚-3-O-芸香糖苷在后,从而对异构体的色谱峰进行相应归属。
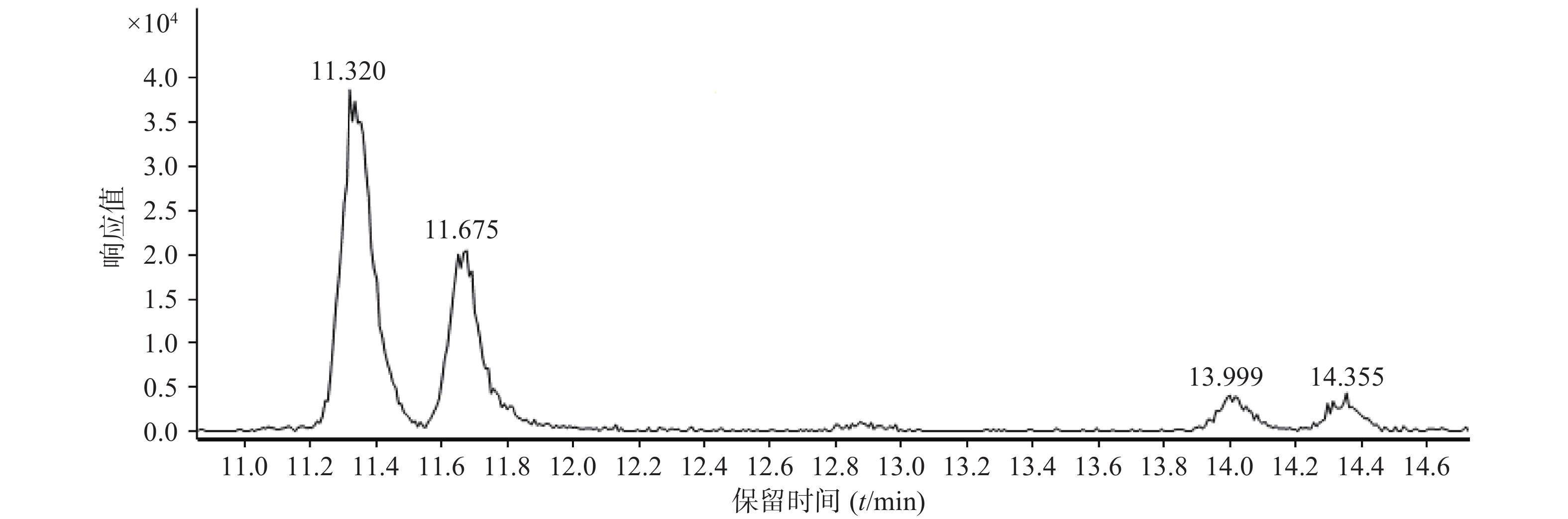
我们还用此方法对同一分子式的一组多个同分异构体进行logP值的排序,以确定这些成分的出峰顺序来进行归属。例如在总离子流图中,第21、22、28、30号峰为一组同分异构体,分别为车前黄酮苷、木犀草素-7-O-葡萄糖苷、异荭草素和三叶豆苷,其结构式如图6所示。在UHPLC-Q-TOF/MS总离子流图中,提取m/z为449.1089的离子,结果见图7。对它们的油水分配系数logP值进行排序,可知该4种同分异构体的出峰顺序,结果如表3所示。
出峰编号 化合物名称 logP值 保留时间(t/min) 21 车前黄酮苷 −0.30±0.64 11.320 22 木犀草素-7-O-葡萄糖苷 −0.09±0.64 11.675 28 异荭草素 1.58±0.88 13.999 30 三叶豆苷 1.95±1.43 14.355 -
本研究采用UHPLC-Q-TOF/MS技术,实现了对中成药复方金钱草颗粒中多成分的快速定性分析,共鉴定出2个苯乙醇苷类、1个低聚糖类、8个酚酸类、27个黄酮类、1个生物碱类、5个萜类、1个脂肪酸类、2个酯类成分(共47个活性成分),该技术为中药复方多成分分析提供了一种有效、可靠的方法,也同样适用于其他中药复方复杂体系的化学成分分析。
由于中药复方化学成分较单味药材更多更复杂,因而同分异构体的情况非常普遍。使用的四极杆复合飞行时间质谱(Q-TOF)在保持了较高的检测灵敏度以及质量准确性的前提下,可以对样本中所有的成分的质荷比进行全谱采集。这种技术使数据采集效率有了极大的提高,更加适用于中药重要复杂体系化学成分的鉴定。
虽然本研究基于复方金钱草颗粒化学成分数据库,利用精确分子量匹配和碎片离子归属策略并辅以软件计算的方法鉴定出了多个化学成分,但仍有大量的同分异构体无法被有效地区分开来,因此,在下一步实验中,使用标准品对照的方法进行更精确地鉴定。
UHPLC-Q-TOF/MS analysis of chemical constituents in compound Jinqiancao granules
doi: 10.12206/j.issn.1006-0111.202105117
- Received Date: 2021-05-26
- Rev Recd Date: 2021-10-28
- Available Online: 2022-03-29
- Publish Date: 2022-03-25
-
Key words:
- compound Jinqiancao granules /
- UHPLC-Q-TOF/MS /
- chemical constituents
Abstract:
| Citation: | ZHANG Zhe, SUN Zhuoran, PAN Pengchao, CHEN Xiaofei, CHAI Yifeng. UHPLC-Q-TOF/MS analysis of chemical constituents in compound Jinqiancao granules[J]. Journal of Pharmaceutical Practice and Service, 2022, 40(2): 146-151, 156. doi: 10.12206/j.issn.1006-0111.202105117 |











 DownLoad:
DownLoad: