-
近年来,随着医疗水平和医疗技术的不断发展,越来越多的儿童侵袭性真菌感染被确诊,尤其在免疫缺陷、血液和肿瘤、器官移植以及需要重症和新生儿监护室的患儿中出现较高的发病率[1-3],引起较高的病死率,或产生严重的后遗症[4]。伏立康唑[5]是第二代三唑类抗真菌药物,有着较广谱的抗菌谱。主要推荐作为侵袭性曲霉菌、对氟康唑耐药的念珠菌及其他不常见的侵袭性病原菌的一线治疗药物。血液稳态谷浓度是对伏立康唑在体内药动学的评价指标,与临床疗效及不良反应有着密不可分的关系。监测伏立康唑在儿童患者的血药浓度,对指导临床安全有效用药具有重要意义。
本研究通过收集2019年1月至12月期间68例使用伏立康唑的住院儿童信息,进行血药谷浓度监测,并对临床有效性和安全性进行分析,以期为真菌感染患儿临床用药提供参考。本研究通过医院伦理委员会的审批(批准号:2019-LDYY-036),所有患儿家属均签署知情同意书。
-
本研究收集2019年1月至12月上海市宝山区罗店医院、上海市儿童医院及上海市同济医院住院治疗的真菌感染患儿病例,共收集到有血药浓度监测的病例68例,其中,男38例(56%),女30例(44%),年龄17天至15岁,详见表1。68例患儿中,6例来自新生儿病区,30例PICU,32例血液病区。感染部位涉及肺部、血液、腹腔、中枢、肠道、泌尿道。按照侵袭性真菌感染诊断标准分为确诊、临床诊断和拟诊[6]。本资料中28例患儿为确诊病例,40例患儿为临床诊断病例。所有案例均存在高危因素,并具有影像学变化。确诊病例即连续3次培养检出同一种真菌,确诊为真菌感染。临床诊断病例的1,3-β-D葡聚糖抗原(G试验)和/(或)半乳甘露聚糖(GM试验)阳性。
年龄 性别 例数(%) 男(%) 女(%) 新生儿 4(11) 2(7) 6(9) 29天~2岁 4(11) 4(13) 8(12) 2~12岁 24(63) 22(73) 46(67) 12~15岁 6(15) 2(7) 8(12) 合计 38(56) 30(44) 68(100) -
纳入标准:诊断为侵袭性真菌感染的儿童;接受伏立康唑治疗或改变剂量后持续用药至少3 d;接受伏立康唑血浆稳态谷浓度监测。排除标准:接受伏立康唑治疗不足3 d;未进行伏立康唑血药浓度监测;肝肾功能不全;缺乏完整的实验室评价指标,不能进行疗效和安全性评价者。
-
注射用伏立康唑(美国辉瑞制药有限公司、晋城海斯制药有限公司),规格:200 mg/瓶,生产批号:Z572301、20210501。应用参考指征:①根据实验室检测结果及药敏试验。②根据临床表现及(G试验)和/(或)(GM试验)阳性,经验性给予抗真菌药物。68例患儿均予以静脉给药,q12h,使用前均告知家长可能的风险并征得家长的同意。
-
研究对象接受伏立康唑治疗或改变剂量后持续用药至少 3 d后检测血清谷浓度。使用全自动二维液相色谱耦合仪LC-100HP(湖南德米特仪器有限公司)进行HPLC法测定药物浓度:血样本采集后静置至自然凝固,4500 r/min离心 5 min,获取血清样本;取 300 μl 血清加入900 μl 乙腈去蛋白溶液,涡旋震荡1 min,15000 r/min离心8 min,待分层完全后,取 1000 μl 上清液加入100 μl 乙腈去蛋白溶液进样,在色谱条件下检测,记录峰面积并计算伏立康唑谷浓度。
-
采用 SPSS 20.0 软件进行统计学分析,计数资料以百分比表示,符合正态分布的计量资料以(
$ \overline{\text{x}}\text{±}\text{s} $ )表示,两样本比较采用独立样本 t 检验,多组间比较采用单因素方差分析,非正态分布以中位数(四分位间距)表示,各组总体水平差异采用 Kruskal-Wallis 法检验,相关性分析采用 Spearman 相关分析,以P<0.05为差异有统计学意义。 -
本案例中28例明确检测到病原菌,其中,10例白色念珠菌,6例热带念珠菌,5例近平滑念珠菌,3例光滑念珠菌,2例曲霉菌,1例挪威假丝酵母菌,1例似平滑念珠菌。伏立康唑治疗真菌感染的患儿,有效58例,有效率85.3%(58/68例),无效10例,无效率14.7%(10/68例)(其中,死亡2例,4例放弃治疗自动出院,4例更换药物)。
-
68 例患儿均静脉给药伏立康唑,使用疗程为7~38 d,平均为(13.2±9.0)d。患儿单次给药剂量3.6~8.0 mg/kg,平均(6.1±1.4) mg/kg。按单次不同给药剂量分为<4.0、4.0~7.0、>7.0 mg/kg 3组。其中,<4.0 mg/kg有6例,有效5例、无效1例;4.0~7.0 mg/kg有44例,有效37例、无效7例;>7.0 mg/kg有18例,有效16例、无效2例,具体见表2。
组别(μg/ml) 例数 有效(%) 无效(%) <4.0 6 5(83.3) 1(16.7) 4.0~7.0 44 37(84.1) 7(15.9) >7.0 18 16(88.9) 2(11.1) 合计 68 58(85.3) 10(14.7) -
根据稳态血清谷浓度值范围分为<1.0、1.0~5.5和>5.5 μg/ml 3组,其中,<1.0 μg/ml有14例,有效10例,无效4例;1.0~5.5 μg/ml有48例,有效44例,无效4例;>5.5 μg/ml有6例,有效4例,无效2例。采用单因素方差分析显示,3组间差异有统计学意义(χ2=5.360, P=0.039),见表3。
组别(μg/ml) 例数 有效(%) 无效(%) χ2 P <1.0 14 10(71.4) 4(28.6) 5.360 0.039 1.0~5.5 48 44(91.7) 4(8.3) >5.5 6 4(66.7) 2(33.3) 合计 68 58(85.3) 10(14.7) -
对68例患儿进行78次谷浓度检测,其中复测10例。复测的病例中,7例为给药剂量上调后复测,3例为给药剂量下调后复测,本研究以第一次谷浓度值为准,复测值不计入其中。不同的给药剂量,检测出不同的谷浓度值。给药<4.0 mg/kg,谷浓度在0.4~3.31 μg/ml (r=0.613,P=0.195);4.0~7.0 mg/kg,谷浓度0.35~7.02 μg/ml (r=0.325,P=0.018);>7.0 mg/kg,谷浓度1.46~12.45 μg/ml (r=0.584,P=0.023),给药剂量与药物浓度之间有相关性,并且3组间谷浓度有统计学差异(F=7.270, P=0.026),见表4。
组别(mg/kg) 例数 谷浓度(μg/ml)
中位数
(四分位间距)r P F P <4.0 6 1.59(0.40~2.76) 0.613 0.195 7.270 0.026 4.0~7.0 53 1.91(0.93~3.63) 0.325 0.018 >7.0 9 3.31(2.04~5.49) 0.738 0.023 -
给药后按照诺氏评估量表[7]评估患儿发生不良反应与伏立康唑的关联性,68例患儿中有10例存在与伏立康唑很可能有关的药物不良反应,主要表现为轻度肝功能损伤[8],其中,2例总胆红素升高,5例(其中,1例为<2岁)谷丙转氨酶(ALT)升高,3例(其中1例新生儿)谷草转氨酶(AST)升高。本案例中未发现神经毒性(视觉障碍、脑病、神经系统疾病)、皮疹等不良反应。其中,低血清谷浓度组中有1例(7.1%)发生ALT升高,2例(33.3%)发生在高谷浓度组中(1例为总胆红素升高,1例为ALT升高),其余7例(14.6%)发生在目标谷浓度组中。
-
儿童感染的真菌类型中,以念珠菌和曲霉菌最为常见,其他有隐球菌、毛霉菌、镰刀菌等[9]。伏立康唑因其抗菌谱广[10]、耐药率低等优点,被多项指南推荐为临床一线抗真菌药物。
-
由于伏立康唑药代动力学的个体差异性较大,中国药理学会建议进行伏立康唑药物浓度监测和基因型检测的个体化用药[11]。美国感染病学会(IDSA)推荐2~12岁儿童无需使用负荷剂量,可直接给予维持量。本研究中的儿童均静脉使用了维持量,无1例使用负荷量及口服给药。美国FDA推荐如下:2~12岁儿童用药剂量为8 mg/(kg·次),q12h。然而在实际使用过程中,考虑患儿的各器官功能,各项实验室指标,及独特的生理特点等因素,临床给药剂量范围有一定的上下浮动。本研究中伏立康唑的给药范围在3.6~8.0 mg/(kg·次),q12h,分为<4.0、4.0~7.0和 >7.0 mg/kg 3种给药剂量。虽然本研究中部分案例给药剂量稍低,但所测得血药浓度在合理的暴露范围内,并未产生较高的治疗失败结果。本研究的给药剂量可以为伏立康唑的儿童用药剂量提供一些参考,实践证明,结合治疗药物浓度监测不仅能提高有效率,同时还能避免药物不良反应的发生。
-
伏立康唑在各国指南中推荐的谷浓度各不相同,浓度下限的范围是0.5~2 mg/L[12-14],浓度上限范围是4~6 mg/L[15-17],目标浓度是1.0~5.5 mg/L。然而以上是成人的谷浓度参考范围,对于儿童的药物浓度推荐的此类研究却较少。本研究按测得的谷浓度值,分成<1.0、1.0~5.5和 >5.5 μg/ml,分别获得不同的有效率,其中,1.0~5.5 μg/ml的谷浓度范围的临床疗效高于另外2组,并且差异有统计学意义(χ2=5.360, P=0.039)。可见,达到伏立康唑的目标谷浓度有助于提高临床有效率(91.7%),低谷浓度影响了临床疗效(71.4%),但更高的谷浓度却未提高有效率(66.7%),反而容易发生不良反应(33.3%)。由此,为确保疗效,降低不良反应的发生,结合我们的研究结论,1.0~5.5 μg/ml可作为儿童伏立康唑的参考血药浓度范围。
-
伏立康唑在治疗真菌感染过程中最为常见的不良反应为肝毒性、神经毒性(视觉障碍、脑病、神经系统疾病)、皮疹等。 考虑到药物的安全性和有效性数据尚不充分,美国FDA推荐2岁以上儿童使用该药物,然而在小婴儿重症感染时,往往会遇到其他抗生素耐药或无效时,结合临床及实验室检查等,权衡利弊后,选择使用伏立康唑。本研究中有14例(14/68,20.6%)儿童<2岁,但只有2例(1例为新生儿,1例为1岁儿童)发生肝功能的异常,经保肝治疗后恢复。同样有6例儿童的谷浓度>5.5 μg/ml,却有2例因高谷浓度而发生药物不良反应。临床医师根据药物浓度结果及肝损伤指标及时下调给药剂量,未产生严重后果。可见,伏立康唑在2岁以下婴儿中有较高的安全性,结合TDM更能减少不良反应的发生。与Park[18]等做的一项随机对照研究结果较相符合,此外,我们的研究结果如王晓晨[19]等建议的那样,治疗窗较窄的伏立康唑,为确保临床疗效,需设计合理的个体化给药方案。
-
本研究中发现虽然给予合理的伏立康唑药物剂量,但测得的谷浓度仍低于<1.0 μg/ml,上调给药剂量后,复测谷浓度仍未出现明显上升。有研究显示[20]伏立康唑在儿童体内的代谢清除率速度较成人更快,更多的研究提示肝脏细胞色素P450同工酶代CYP2C19[21]对药物在体内的暴露、清除、代谢等起着至关重要的影响。而CYP2C19的基因型对伏立康唑的代谢快慢起到关键的作用,由此可在一定程度上解释合理的给药剂量却产生较低药物暴露量的原因。本研究中伏立康唑的总体安全性尚可,但仍需提醒,临床医师需要评估病情,根据循证医学、病原菌检测及药物敏感实验,结合医学伦理问题,权衡患儿风险及收益,可以谨慎使用。
本研究存在一定的局限性,只监测了伏立康唑的血药浓度,收集了给药剂量、肝肾功能等数据,初步得出了一些结论,并未深入研究目前热门的药物基因对伏立康唑代谢的影响。因样本量小,亦未对2岁以内的儿童进行详细的研究。总之,开展儿童使用伏立康唑的血药浓度监测,对药物的有效性和安全性起到一定的指导意义。
Study on the relationship between blood concentration and efficacy of voriconazole in the treatment of pediatric invasive fungal infection
doi: 10.12206/j.issn.1006-0111.202106075
- Received Date: 2021-06-13
- Rev Recd Date: 2021-12-08
- Available Online: 2022-07-27
- Publish Date: 2022-07-25
-
Key words:
- voriconazole /
- invasive fungal infection /
- children /
- drug concentration /
- clinical efficacy
Abstract:
| Citation: | GUI Mingzhu, LI Jing, XIE Xiaotian, LI Zhiling. Study on the relationship between blood concentration and efficacy of voriconazole in the treatment of pediatric invasive fungal infection[J]. Journal of Pharmaceutical Practice and Service, 2022, 40(4): 359-363. doi: 10.12206/j.issn.1006-0111.202106075 |








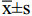
 DownLoad:
DownLoad: