-
昆仙胶囊是由昆明山海棠、淫羊藿、枸杞子、菟丝子四味中药组成的复方制剂,是国家“九五攻关”项目成果转化的中药六类新药,具有细胞因子拮抗、免疫抑制、抗炎镇痛等作用,临床上用于风湿性关节炎等免疫性疾病的治疗,疗效显著[1-5],这可能与各单味药材中富含生物碱、萜类及黄酮类等多种抗炎镇痛的化学成分密切相关[6-9]。雷公藤甲素为君药昆明山海棠中的二萜内酯类毒性效应成分,且有效剂量和中毒剂量极为相近[10-11],因此,为保证患者用药的安全性及有效性,有必要对昆仙胶囊中雷公藤甲素的含量进行限定。目前,昆仙胶囊现有的质量控制标准中仅对组方中的淫羊藿和枸杞子进行薄层鉴别,含量测定项下则监测雷公藤甲素和淫羊藿苷的含量,但通过实验发现雷公藤甲素的含量测定方法仍然存在前处理方法复杂,含量低,重现性及检测成分专属性差等亟待改进的问题,同时,原检验项目的设置也不够全面,难以对昆仙胶囊实现整体性质量控制。因此,本实验采用薄层色谱法补充建立方中所有药味的薄层鉴别方法,并改良了昆仙胶囊中微量毒效成分雷公藤甲素的含量测定方法,为提升该制剂的质量标准研究提供实验依据。
-
Linomat-Ⅵ薄层色谱自动点样器、Reprostar3薄层色谱摄像仪(瑞士CAMAG 公司);Agilent1260高效液相色谱仪,包括输液泵、自动进样器、柱温箱、二极管阵列检测器(美国Agilent公司);BP211D十万分之一电子天平(德国Sartorius公司);KQ-250DB数控超声波清洗器(昆山市超声仪器有限公司)。
-
雷公藤甲素(含量:99.8%,批号:111567-201404)、淫羊藿苷(含量:94.2%,批号:111737- 01516)、金丝桃苷(含量:94.9%,批号:111521-201809)对照品,以及对照药材:昆明山海棠(批号:121203-201503)、淫羊藿(批号:121632-201502)、菟丝子(批号:121232-201403)均购自中国食品药品检定研究院;21批昆仙胶囊以及各阴性样品均由广州白云山陈李济药厂有限公司提供,胶囊的批号分别为:K31002、K31005、K31006、K31007、K31008、K31009、K31010、K31011、K31012、K31013、K31014、L31001、L31002、L31003、L31004、L31005、L31006、L31007、L31008、L31009、J31002,分别编号为S1~S21。乙腈、甲醇、甲酸均为色谱纯,水为杭州娃哈哈纯净水,其余试剂均为分析纯。
-
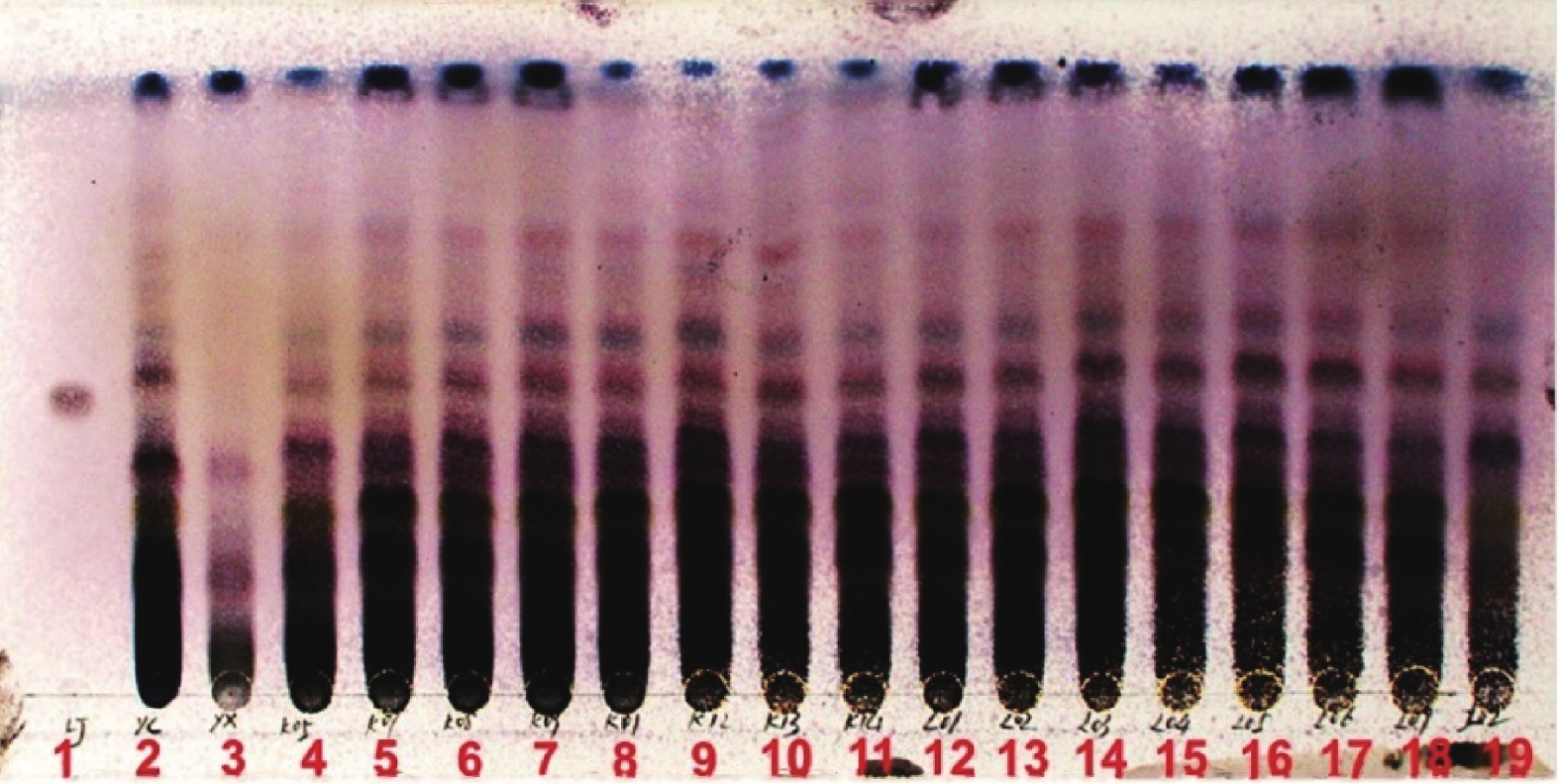
取昆仙胶囊内容物6 g,放入500 ml具塞锥形瓶中,加入300 ml甲醇,超声30 min,过滤,将滤液减压浓缩至干,残渣加40 ml水溶解,二氯甲烷萃取2次,每次40 ml,并于萃取过程中加5 ml饱和NaCl水溶液,合并二氯甲烷萃取液,减压浓缩至干,残渣加少量甲醇溶解,加1 g硅胶(200~300目)拌样,称4 g硅胶装入直径为2 cm的柱子,上柱。先用120 ml石油醚-乙酸乙酯(3∶1)洗脱,再以120 ml石油醚-乙酸乙酯(2∶1)洗脱,收集洗脱液,减压浓缩至干,残渣加入2 ml甲醇溶解,作为供试品溶液。另取昆明山海棠对照药材10 g,同法制备残渣后,加2 ml甲醇溶解,作为对照药材溶液。再取雷公藤甲素对照品加甲醇制成1 ml含0.5 mg的溶液,作为对照品溶液。按照薄层色谱法(通则0502)试验,分别吸取对照品溶液5 μl,对照药材溶液及供试品溶液各10 μl,分别点于同一高效硅胶G薄层板上,以二氯甲烷-丙酮(12∶1,V/V)为展开剂,展开,取出,晾干,喷以2%香草醛的10%硫酸乙醇溶液,105ºC加热至斑点显色清晰。日光下,供试品色谱中,在与对照药材和对照品色谱相应的位置上,显相同颜色的斑点,阴性无干扰(见图1)。
-
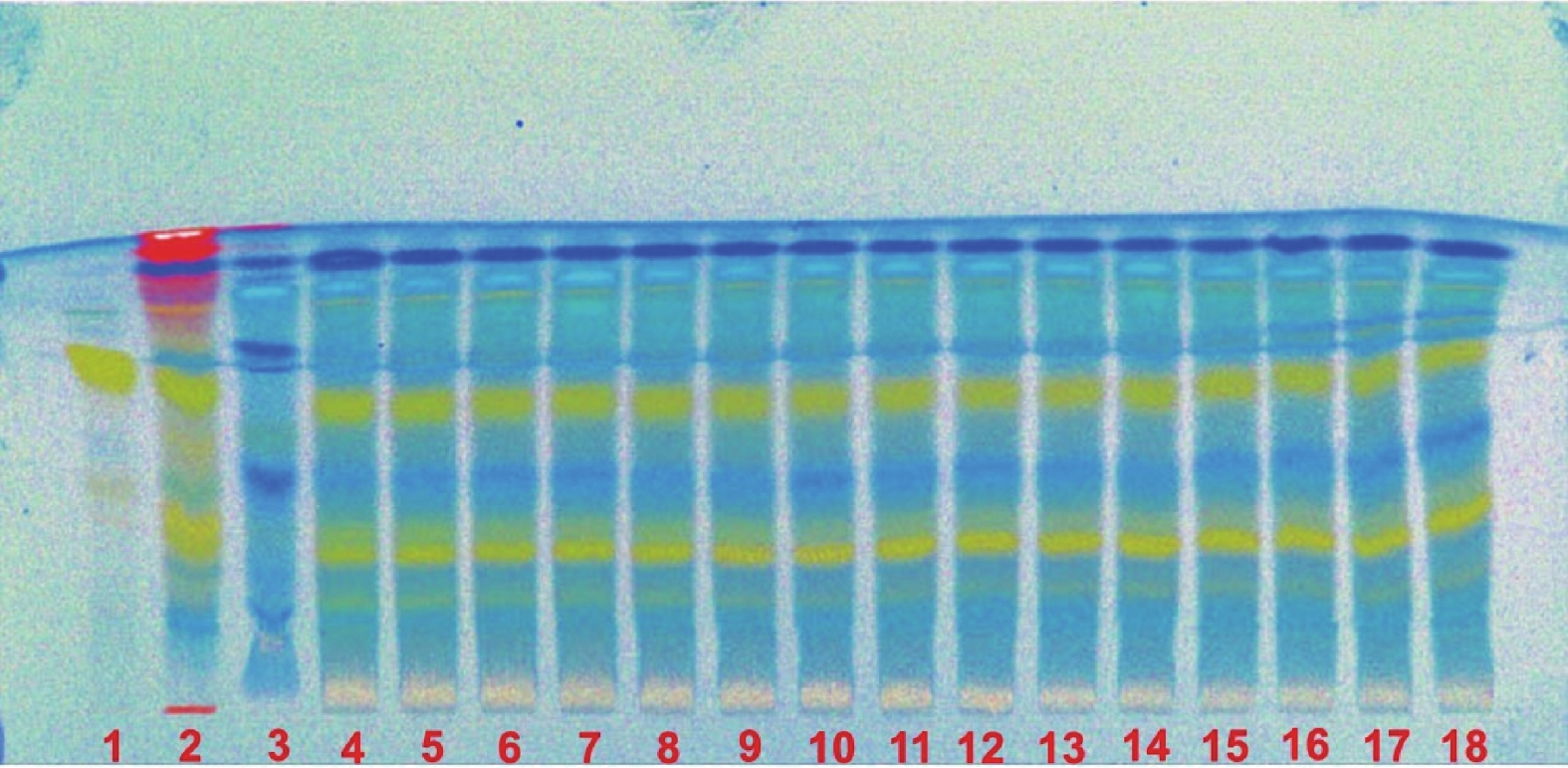
取本品内容物50 mg,加70%乙醇2 ml溶解,滤过,作为供试品溶液。另取淫羊藿对照药材0.5 g,加乙醇10 ml,40~60℃温浸30 min,滤过,滤液蒸干,残渣加乙醇l ml使溶解,作为对照药材溶液。再取淫羊藿苷对照品加乙醇制成1 ml含0.5 mg的溶液,作为对照品溶液。按照薄层色谱法(通则0502)试验,吸取上述3种溶液各5 μl,分别点于同一高效硅胶G薄层板上,以二氯甲烷:甲醇:水:甲酸(7∶3∶1∶0.2)的下层溶液为展开剂,展开,取出,晾干,喷以三氯化铝试液,烘干,置紫外光灯(365 nm)下检视,供试品色谱中,在与对照药材色谱和对照品色谱相应的位置上,显相同颜色的荧光斑点,阴性无干扰(见图2)。
-
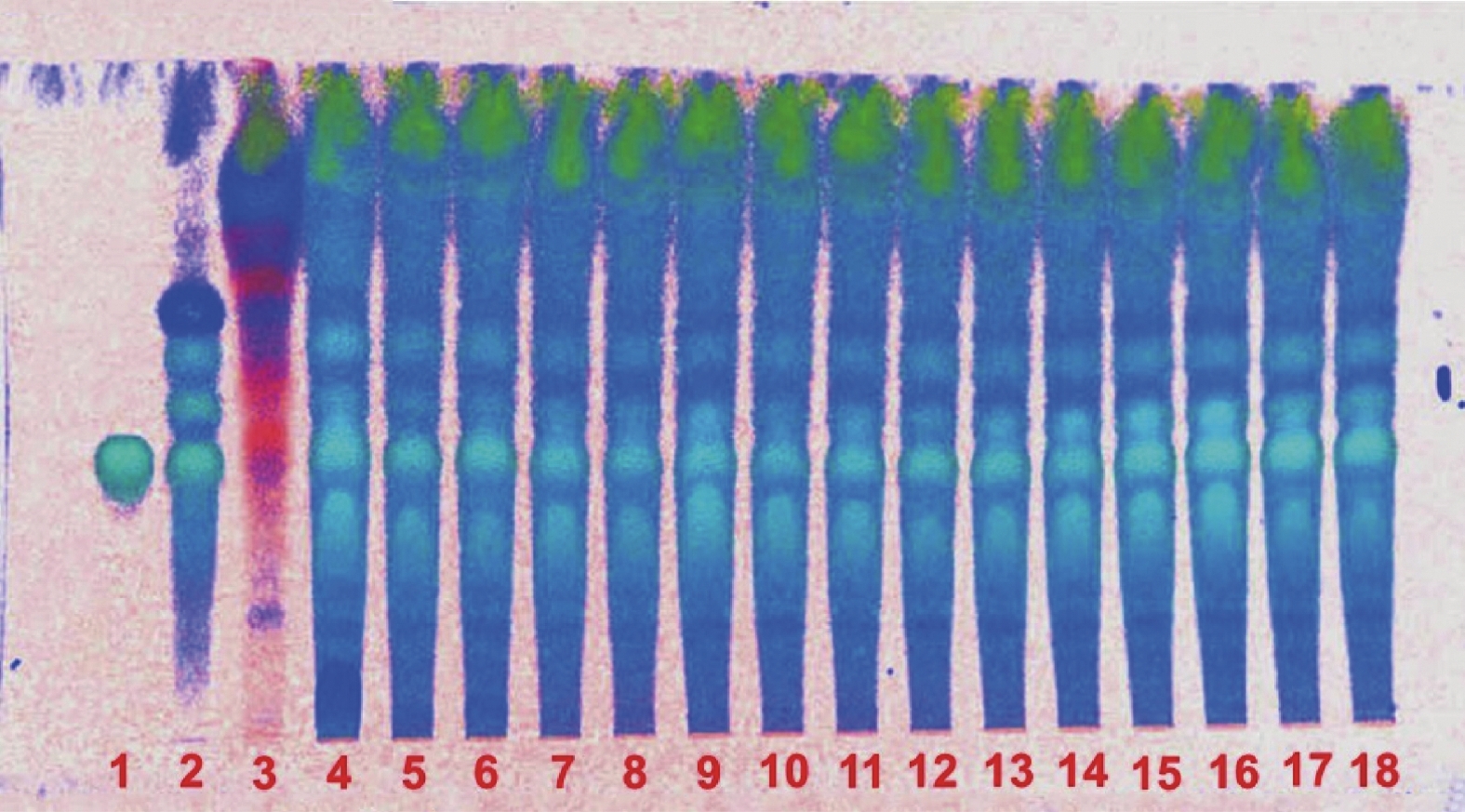
取本品内容物50 mg,加70%乙醇2 ml溶解,滤过,作为供试品溶液。另取菟丝子对照药材0.5 g,加乙醇40 ml,超声30 min,滤过,滤液浓缩至5 ml,作为对照药材溶液。再取金丝桃苷对照品加乙醇制成1 ml含0.3 mg的溶液,作为对照品溶液。照薄层色谱法(通则0502)试验,吸取上述对照药材及金丝桃苷对照品溶液各2 μl,供试品溶液5 μl,分别点于同一聚酰胺薄膜板上,以乙酸乙酯:甲醇:水:甲酸(12∶2∶1∶1)为展开剂,展开,取出,晾干,喷以三氯化铝试液,105 ºC加热至斑点显色清晰,置紫外光灯(365 nm)下检视,供试品色谱中,在与对照药材色谱和对照品色谱相应的位置上,显相同颜色的荧光斑点,阴性无干扰(见图3)。
-
色谱柱:Agilent ZORBA SB-C18(4.6 mm×250 mm, 5 μm);流动相:乙腈(C)-0.1%甲酸水溶液(A);梯度洗脱:0~10 min,15%→23% C;10~35 min,23%→26% C;35~40 min,26%→26% C;40~41 min,26%→98% C;41~52 min,98%→98% C;检测波长:220 nm(紫外检测时间40 min);柱温:30 ℃;流速:0.8 ml/min;进样量:10 μl。
-
取雷公藤甲素对照品,加甲醇制成每毫升含502 μg雷公藤甲素溶液,作为对照品储备溶液。
-
取昆仙胶囊内容物约6 g,精密称定,其余步骤同“2.1.1”项下的方法,制得供试品溶液。
-
取缺昆明山海棠的阴性样品适量,按照“2.1.1”项下的方法操作,制得阴性对照溶液。
-
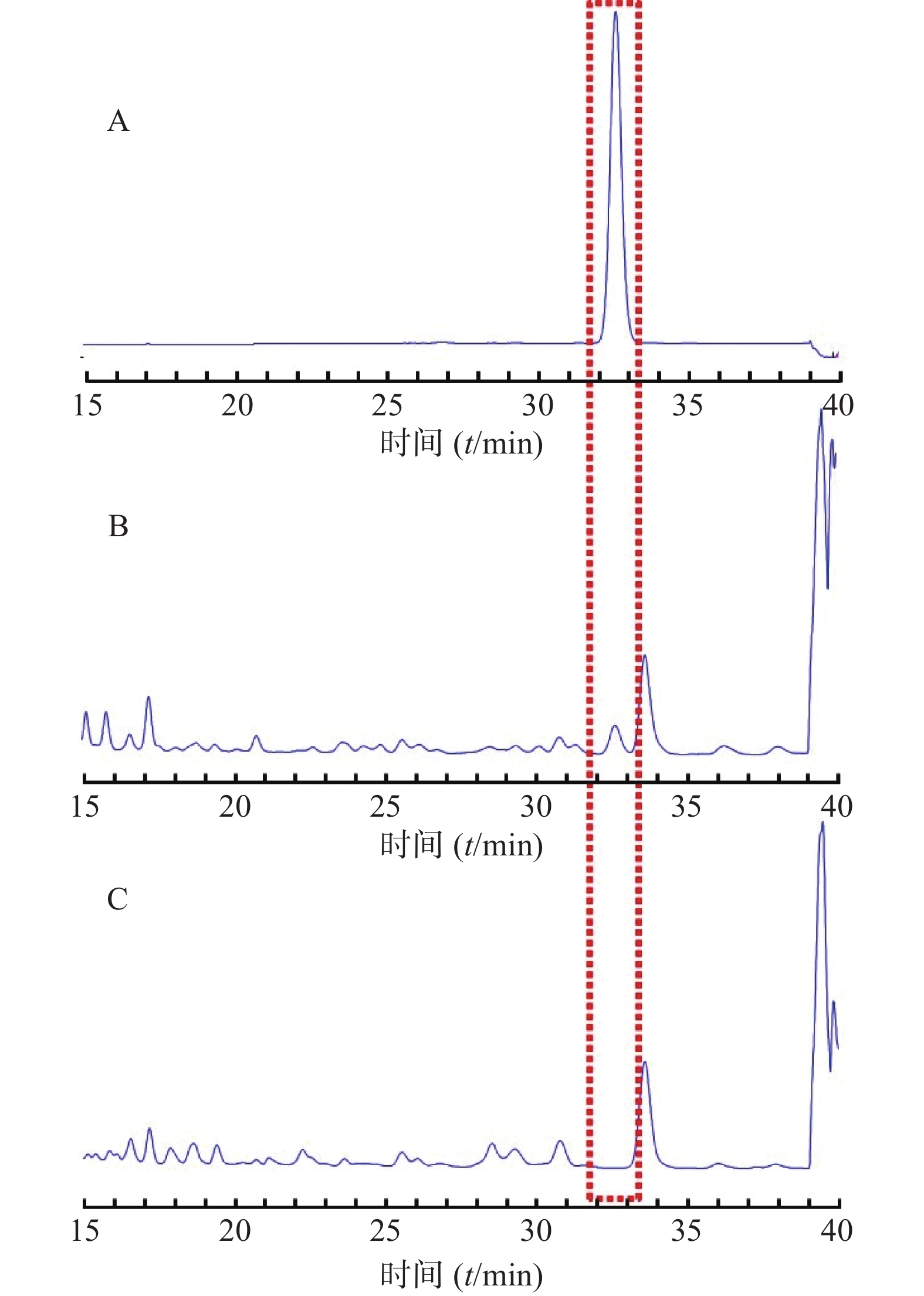
分别精密吸取对照品溶液、供试品溶液、阴性对照溶液各10 μl,按“2.2.1”项下色谱条件分析,结果显示,其与相邻色谱峰的分离度大于1.5,拖尾因子在0.95~1.10,理论塔板数在5000以上,且阴性样品在各色谱峰的位置上未见干扰,说明该方法专属性良好,其HPLC图谱见图4。
-
将雷公藤甲素对照品储备液用甲醇稀释制成每毫升含502.00、401.60、200.80、100.40、50.20、40.16 μg的系列溶液,分别吸取10 μl注入液相色谱仪,记录色谱图,以峰面积Y对质量浓度X(μg/ml)进行线性回归,得回归方程:Y=24.459X+347.82,r= 0.999 5,线性范围为40.16 ~502.00 μg/ml。
-
取雷公藤甲素对照品溶液(100.4 μg/ml),进样10 μl,连续进样6次,测定峰面积,结果显示雷公藤甲素的平均峰面积为2813.93,RSD为0.19%,表明仪器精密度良好。
-
按照供试品溶液制备方法,平行制备6份供试品溶液,分别进样10 μl,测定峰面积,结果表明,雷公藤甲素的平均含量为0.0097 mg/粒,RSD为3.57%,表明该方法重复性较好。
-
取某一批次(L31008)样品,制备成供试品溶液,分别于0、3、6、9、12、24、36、48 h分别进样测定雷公藤甲素的峰面积,结果显示雷公藤甲素平均峰面积为2647.16,RSD为1.53%,说明供试品溶液在48 h内稳定。
-
分别精密称取6份已知含量的样品约6 g,精密称定,置于具塞锥形瓶中,按照接近1∶1的量计算所需添加的雷公藤甲素对照品溶液,加入到样品中,按“2.2.3”项下方法平行制成6份供试品溶液,进样10 μl,记录峰面积,结果显示雷公藤甲素的平均回收率为98.12%,RSD为8.25%,说明该方法准确度较好,计算结果见表1。
样品量(m/mg) 加入量(m/mg) 测得量(m/mg) 回收率(%) 平均回收率(%) RSD
(%)0.097 6 0.097 9 0.188 7 92.98 98.12 8.25 0.097 6 0.097 9 0.181 8 85.92 0.097 6 0.097 9 2.050 5 109.71 0.097 6 0.097 9 1.937 0 98.11 0.097 6 0.097 9 1.972 9 101.78 0.097 6 0.097 9 1.957 8 100.23 -
分别取每批昆仙胶囊粉末约6 g,精密称定,按“2.2.3”项下的方法制成供试品溶液,每批样品平行制备2份,每份进样3针,进样量10 μl,记录峰面积,计算结果见表2。
批号 平均含量(mg/粒) 批号 平均含量(mg/粒) S1 0.010 3 S12 0.011 7 S2 0.009 2 S13 0.011 2 S3 0.009 1 S14 0.013 3 S4 0.005 9 S15 0.008 8 S5 0.011 7 S16 0.012 6 S6 0.009 0 S17 0.012 2 S7 0.006 5 S18 0.007 0 S8 0.007 6 S19 0.009 8 S9 0.011 6 S20 0.008 8 S10 0.010 2 S21 0.011 8 S11 0.008 4 -
本研究采用TLC法补充了昆仙胶囊全药味的鉴别方法,各项方法重复性好,专属性强,且阴性无干扰。与原标准相比,改良后的淫羊藿药材的薄层鉴别方法所获得的斑点分离度更佳,比移值适中;新增的昆明山海棠药材薄层法中点状点样优于条带状点样,喷以2%香草醛的10%硫酸乙醇溶液显色方式优于10%硫酸乙醇溶液显色;新增的菟丝子药材薄层鉴别中,按照2020版中国药典中菟丝子的鉴别方法以甲醇-冰醋酸-水(4∶1∶5)为展开剂时[12],所获得的目标斑点分离度及成点性均差,而以乙酸乙酯-甲醇-水-甲酸(12∶2∶1∶1)为展开剂时,所获目标斑点的成点性及分离度均得到有效改善。但由于所测成分化学性质相差较远,尚无法实现“一板多测”。
-
由于本品成分复杂,雷公藤甲素又属于微量成分,需对昆仙胶囊的前处理条件进行优化。首先,我们参考了厂家的前处理方法,即利用甲醇提取蒸发浓缩后,直接过氧化铝-硅胶柱纯化,结果得到的目标化合物含量较低,操作方法可重复性差,且液相色谱中雷公藤甲素分离度较差,易受其他化合物干扰。为此,我们又尝试通过甲醇提取,再多次萃取富集纯化的方法,均不能实现有效的测定。进而,我们结合厂家的方法,对我们的方法进行了优化,最终确定为甲醇超声提取后,用二氯甲烷萃取,然后通过硅胶柱再次富集和纯化目标成分,最终不仅提高了HPLC法检测雷公藤甲素含量的上限,且大大减少了其他化合物对目标色谱峰的干扰。整个前处理过程,还比较了不同的提取溶剂和萃取溶剂的效果,结果均不如本法。
-
由于样品中其他黄酮类成分的含量相对较高,按照现行部颁标准中的液相方法测定目标成分时,容易出现色谱柱对目标成分的分离效果差,含量测定结果的重复性、重现性不理想,RSD较大等问题。为此,本实验又对定量测定雷公藤甲素含量的色谱条件进行了优化,主要比较了不同色谱柱(Diamonsil C18、Agilent ZORBA SB-C18、Amethyst C18及Waters SunFireTm C18),不同流动相(乙腈-0.1%甲酸水溶液、乙腈-0.2%甲酸水溶液、乙腈-0.1%磷酸水溶液、乙腈-水、甲醇-0.1%甲酸水溶液),不同柱温(25、30、35、40 ℃)及不同体积流量(0.8、0.9、1.0 ml/min)对雷公藤甲素色谱峰的影响,最终以Agilent ZORBA SB-C18为色谱柱、乙腈-0.1%甲酸水溶液为流动相、柱温为30 ℃及流速为0.8 ml/min时,目标峰分离完全,效果最佳。
综上,本文建立的昆仙胶囊薄层鉴别及雷公藤甲素含量测定的方法,不仅弥补了昆仙胶囊全药味薄层鉴别的研究,同时也解决了现行标准中雷公藤甲素难测定,重复性差等难点,为完善昆仙胶囊的质量评价提供了依据。
Improvement of the quality standard for Kunxian capsules
doi: 10.12206/j.issn.1006-0111.202106102
- Received Date: 2021-06-18
- Rev Recd Date: 2021-10-19
- Available Online: 2022-03-29
- Publish Date: 2022-03-25
-
Key words:
- Kunxian capsules /
- TLC /
- triptolide /
- HPLC /
- quality standards
Abstract:
| Citation: | XU Chunfang, LI Zhexuan, ZHOU Luoying, ZHANG Ni, YANG Hong, TAO Xia. Improvement of the quality standard for Kunxian capsules[J]. Journal of Pharmaceutical Practice and Service, 2022, 40(2): 152-156. doi: 10.12206/j.issn.1006-0111.202106102 |









 DownLoad:
DownLoad: