-
肿瘤RNA是目前已知肿瘤疫苗的来源之一[1-2]。先前的研究表明,从CT26结肠癌细胞提取的肿瘤RNA可以通过增强机体抗肿瘤免疫来抑制肿瘤生长[3],但由于肿瘤RNA易降解、不稳定等特性,需要载体对其包裹后实施注射,从而发挥其抗肿瘤作用[4]。研究证实,纳米脂质体载体具有较强的稳定性,可有效地包裹RNA,并在体内和体外递送核酸,发挥核酸诱导或抑制细胞表达的作用[5-7]。然而,机体不同部位对药物的吸收情况不同,因此,导致药物吸收转化的效果产生差异。为了比较不同部位注射对药物治疗效果的影响,本研究从CT26结肠癌细胞中提取肿瘤RNA,并使用纳米脂质体对其进行包裹制备成纳米脂质体疫苗,分析了不同部位注射肿瘤RNA纳米脂质体疫苗所产生的增强机体抗肿瘤反应的情况。
-
R205B旋转蒸发器(上海申科科技有限公司);610000-1EA型薄膜挤出器(美国Avanti公司);必能信Sonifier®450D超声波粉碎仪(美国必能信公司);马尔文粒径仪(英国马尔文仪器公司);透射电子显微镜 JEM2100F(日本JEOL公司);染色封片工作一体机(胶带)Prisma+ Film(日本樱花公司)。
-
(2,3-二油酰基-丙基)-三甲胺硫酸盐((N-1-(2, 3-dioleoyloxy) propyl)-N,N,N-trimethylammoniumethyl sulfate, DOTAP)(美国Avanti Polar Lipids公司);二硬脂酰基磷脂酰乙醇胺-聚乙二醇-羧基(1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(carboxy(polyethylene glycol)-2000) (sodium salt), DSPE-PEG(2000) Carboxylic Acid)(美国Avanti公司);鱼精蛋白、小牛胸腺DNA(美国Sigma公司);胆固醇(上海麦克林生化科技有限公司);氯仿(中国医药集团有限公司);DEPC水(碧云天生物技术有限公司);TRIzol RNA 分离试剂(美国Thermo公司);CT26小鼠结肠癌细胞系(中国科学院典型培养物保藏委员会细胞库),SPF 级 BALB/c 雄性小鼠(上海吉辉实验动物饲养有限公司),饲养于屏障系统中。
-
首先取胆固醇、DOTAP两种材料的氯仿溶液,将二者以1∶1 (m∶m)的比例加入到250 ml的茄型瓶中,随后再加入4 ml氯仿溶液。在真空、45 ℃及100 r/min条件下旋转蒸发45 min,再加入3 ml DEPC水,在45 ℃及100 r/min条件下旋转蒸发45 min,获得脂质体混悬液。先进行超声20 min,再先后通过聚碳酸酯膜(100、50 nm),反复挤出各20次,即得DOTAP/Chol纳米脂质体。
-
取长满培养皿的CT26细胞,倒去培养基,加入3 ml无菌PBS溶液,并轻晃几下进行清洗,然后按照TRIzol™ Reagent 操作说明提取RNA。提取完成后,将核酸溶液用NanoDrop 2000测A值、浓度和纯度。将检测完成后的核酸溶液分装至1.5 ml EP管中,随后放入液氮中保存。
-
准备DOTAP/Chol 脂质体疫苗63 μl,鱼精蛋白12 μl(2 μg/μl)和DEPC水10 μl,将上述材料混合,于室温放置 10 min,得到DOTAP/Chol 脂质体和鱼精蛋白的混合物,在45~50 ℃的范围内水浴加热1~2 min。准备肿瘤RNA 45 μl(0.5 μg/μl),小牛胸腺 DNA 1.2 μl(10 μg/μl)和DEPC 处理水30 μl,将以上材料混合,并室温放置10 min,得到肿瘤RNA与小牛胸腺DNA 的核酸聚合物,将DSPE-PEG(2000) Carboxylic Acid 3.2 μl(10 μg/μl)加入到 RNA/DNA 聚合物中,在45~50 ℃的范围内水浴加热1~2 min,得到RNA/DNA/PEG 复合物。将纳米脂质体/鱼精蛋白复合物加入到 RNA/DNA/PEG 复合物中,常温放置 10 min。最后将混合物放入 55 ℃的环境中静置10 min,然后在常温环境中静置 10 min,即可得肿瘤RNA纳米脂质体疫苗。
-
稀释肿瘤RNA纳米脂质体疫苗至适当浓度,取1 ml进行粒径检测。选择干净的样品池,取肿瘤RNA纳米脂质体疫苗溶液小心加入,在加入的同时避免气泡产生。把样品池放入进样样品槽中,然后进行粒径检测。对于脂质体放置稳定性试验,将脂质体放置于4 ℃,在第1、3、5、7天取样测量粒径。
-
将10 μl的脂质体疫苗溶液缓慢滴加在铜网上,在室温下彻底风干。随后滴加2%磷钨酸溶液,并使用滤纸吸干多余液体,后在室温下彻底干燥。之后将检测铜网放置在透射电镜下进行观察。
-
实验开始后,取对数生长期的CT26细胞,计数为1×106/ml,在小鼠右侧背部近腋窝处接种,每只小鼠皮下接种CT26细胞100 μl(1×105个/只),构建小鼠结肠癌移植瘤模型。接种1周后按照实验方案接种相应脂质体疫苗溶液,频率为每7 天一次,一共3次。其中,PBS 组(n=5)的小鼠进行常规饲养,每只小鼠腹腔注射200 μl的 PBS 作为空白对照。肿瘤RNA纳米脂质体疫苗腹腔注射组(n=5)的小鼠进行常规饲养,每只小鼠腹腔注射200 μl的肿瘤RNA纳米脂质体疫苗,注射频率为每7 天一次,共注射 3 次;肿瘤RNA纳米脂质体疫苗皮下注射组(n=5)的小鼠进行常规饲养,每只小鼠左侧皮下注射200 μl的肿瘤RNA纳米脂质体疫苗,频率为每7 天一次,共注射3 次;当肿瘤体积达到2 000 mm3时处死小鼠,并提取实验小鼠各项指标进行分析。
-
动物实验结束后,将小鼠处死,之后将移植瘤肿瘤团块完全剥离,小心去除肿瘤周围的结缔组织和血管,使用PBS浸泡清洗干净后,使用干净滤纸将水吸干,浸没置于4%多聚甲醛中以4 ℃保持。同时,将各组实验小鼠处死后,将心、肝、脾、肺、肾完整剥离,去除结缔组织和血管,使用PBS浸泡清洗干净后,使用干净滤纸将水吸干,浸没置于4%多聚甲醛中以4 ℃保持。将需要处理的组织块放入合适的耐液氮的冰冻小盒中,将冰冻小盒缓缓放入液氮中,使其彻底浸没在液氮中,浸没约20 s左右,使组织块能够彻底冰冻成块。组织块冰冻成块后,取出冰冻块,快速使用已涂抹有冰冻包埋剂的塑料薄膜进行包封,然后将已经包封好的组织块和切片冻头放入冰箱进行保存备用,冰箱温度设置为−80 ℃。按照冰冻切片机器操纵要求进行冰冻切片,切片厚度控制在20~30 μm左右,然后,将切片样品放入−20 ℃的冰箱进行保存。使用染色封片工作一体机(胶带)Prisma+ Film,按照操作说明进行染色及封片操作。使用数字切片扫描系统 PRECICE 500进行图像采集分析。
-
所有数据均以(
$\bar x $ ±s)表示,统计分析软件使用GraphPad Prism Version 9 (GraphPad, La Jolla, CA, USA),组间差异采用t检验,P<0.05时表示差异有统计学意义。 -
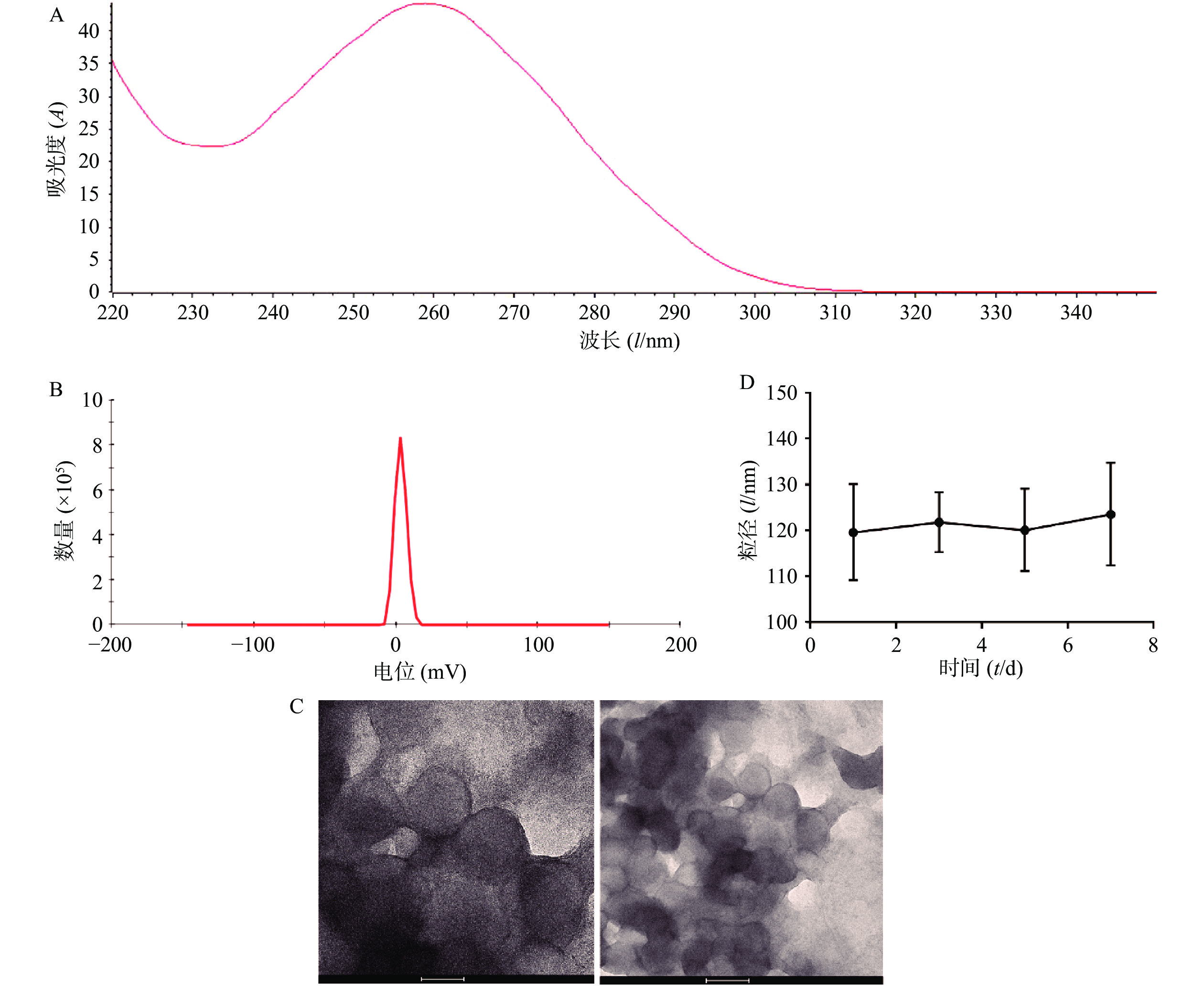
将从CT26细胞中提取的RNA使用NanoDrop 2000进行检测,OD260/OD280为2.04(图1A),OD260/OD230为1.96,符合RNA需达到OD260/OD280>2.0,1.8<OD260/OD230<2.2的要求,且浓度达到1762.1 ng/ml,满足后续实验需要。制备肿瘤RNA纳米脂质体疫苗,通过马尔文激光粒度测定仪对肿瘤RNA纳米脂质体疫苗进行了电位检测,结果显示其Zeta电位为(3.39±0.56)mV(图1B),可以降低脂质体疫苗的聚合从而增加其稳定性。通过透射电子显微镜检测发现,我们制备的肿瘤RNA纳米脂质体疫苗形态规则、呈球形、分布均匀,且粒径大小为(120.0±12.1)nm (图1C)。通过测量不同时间的脂质体疫苗的粒径大小,结果发现放置在4 ℃冰箱的脂质体在7 d内粒径基本维持在120 nm,脂质体放置稳定性良好(图1D)。
-
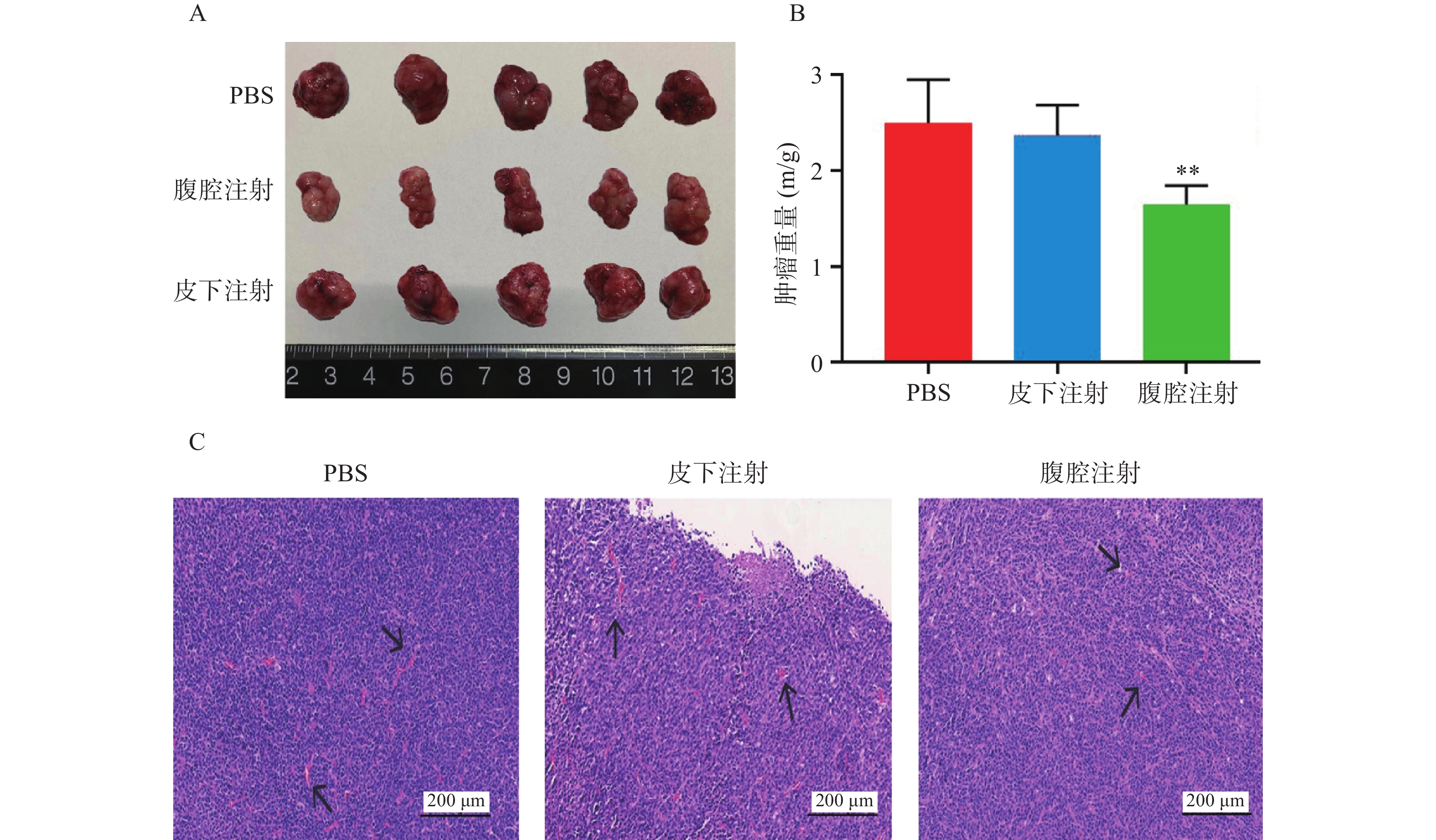
通过完整剥离各组织移植瘤,我们可以直观看到,腹腔注射组比PBS空白对照组、皮下注射组其肿瘤体积更小(图2A)。同时,对各组移植瘤进行称重测量比较(P<0.01,图2B),腹腔注射组比PBS组及皮下注射组平均移植瘤重量更小,肿瘤生长抑制更加明显。由于结肠癌的大小与肿瘤微血管密度呈正相关性,通过对各组移植瘤H&E染色(图2C),发现与PBS组相比,腹腔注射组和皮下注射组在同一视野下肿瘤微血管密度更小,其中,腹腔注射组微血管密度最小。
-
通过完整剥离各组脾脏,我们发现腹腔注射组小鼠的脾脏大于PBS组和皮下注射组(图3)。脾脏是机体重要的免疫器官,在机体抗肿瘤免疫反应中,其大小在一定程度上反应了机体抗肿瘤免疫反应应答强度和免疫水平。因此腹腔注射增强肿瘤RNA的机体抗肿瘤免疫反应的效果更加明显。
-
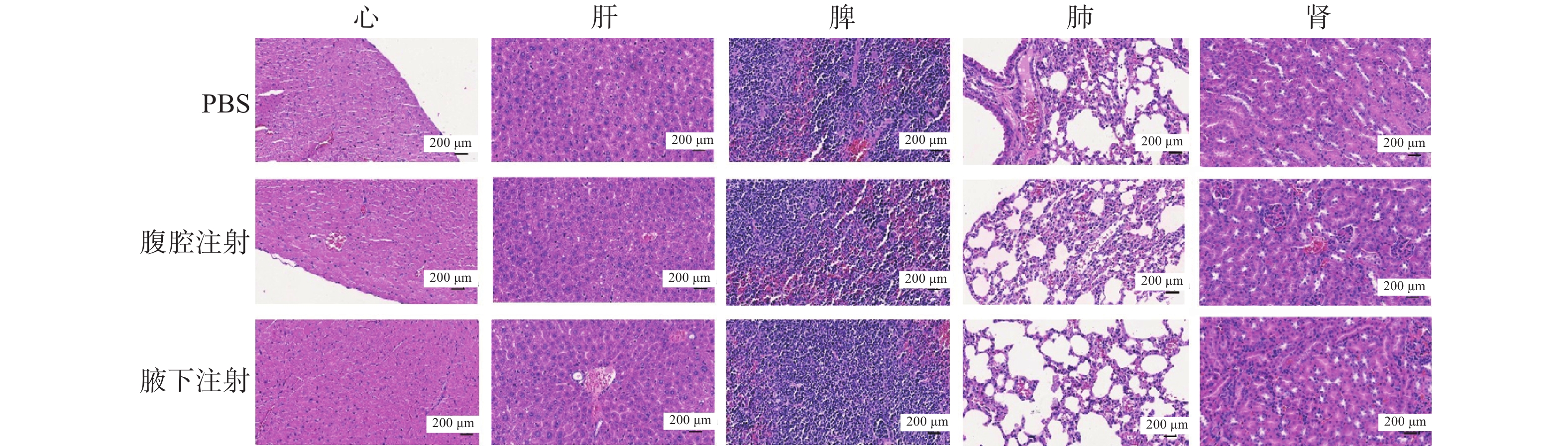
实验结束后,完整剥离小鼠的心、肝、脾、肺、肾等主要脏器并进行H&E染色(图4),结果显示,各组的主要脏器中没有出现异常的组织学改变,肿瘤RNA对机体毒性影响很小,安全性较高。
-
根据2020年全球统计的数据,1930万新增癌症病例中,结肠直肠癌占10.0%,位居第三。在近1000万癌症死亡相关病例中,结肠直肠癌占了9.4%,排在第二位[8]。同时,近10年间,结直肠癌的发病率在我国呈直线上升趋势。因此,我们迫切需要制订新的策略以治疗结直肠癌[9]。研究表明,肿瘤RNA携带有特异性肿瘤抗原,其肿瘤抗原无需检查,并且RNA序列进入宿主基因组内的危险性很小,是优质的肿瘤疫苗来源。此外,有进一步的研究证明,从CT26结肠癌细胞提取的肿瘤RNA可通过增强生物体抗肿瘤免疫来抑制结肠癌生长[1-3]。因此,不同部位注射负载肿瘤RNA的纳米脂质体疫苗的抗肿瘤效果值得进一步探讨,为其后续的进一步应用提供理论基础。
纳米脂质体疫苗注射途径种类较多,如静脉注射、动脉注射、肌内注射、皮下注射、腹腔注射等方式[10-13],研究证明,通过不同部位注射药物,其生物利用度也不同。本研究对移植瘤小鼠进行了不同部位肿瘤RNA纳米脂质体疫苗注射,并分析比较不同部位注射所产生的影响,结果显示,腹腔注射可以抑制肿瘤生长,值得注意的是,腹腔注射后的肿瘤更小、肿瘤微血管密度最低[14-15],因此,腹腔注射抑制肿瘤生长的效果更加显著。根据相关研究,如果药物注射部位周围血液循环及淋巴液循环丰富,那么药物吸收路径较短,较少受到各种影响因素的干扰,会表现出更好的治疗效果,而腹腔中血液循环和淋巴循环分布较皮下更为丰富,因此腹腔注射表现出更好的治疗效果[16-18]。
此外,在本研究中,负载肿瘤RNA的纳米脂质体疫苗的形状为球型体,粒径大小为(120.0±12.1)nm,Zeta电位为(3.39±0.36)mV。实验提示,肿瘤RNA纳米脂质体疫苗组能够引起机体免疫器官脾脏增大[19],并和治疗效果呈正相关。同时在各治疗组及对照组的实验结束后,小鼠各个重要脏器未发生病理改变。所以腹腔注射是更适合肿瘤RNA纳米脂质体疫苗药物注射的途径,这与腹腔血液循环和淋巴循环更加丰富有关,使纳米级的肿瘤RNA纳米脂质体疫苗能够更好更高效地被机体自身吸收利用,进而能够更有效地增强机体自身抗肿瘤免疫反应,并更有力地抑制肿瘤生长及发展。
综上,本研究对同等大小移植瘤小鼠进行了不同部位负载肿瘤RNA的纳米脂质体疫苗注射的动物治疗实验。实验结果显示,相较于皮下注射,腹腔注射能够更加有效地提高机体抗肿瘤免疫反应并抑制肿瘤生长。
Effect of different injection approaches of tumor RNA nanoliposome vaccine on the growth of colon cancer
doi: 10.12206/j.issn.1006-0111.202108094
- Received Date: 2021-08-19
- Rev Recd Date: 2021-10-25
- Available Online: 2021-12-27
- Publish Date: 2021-11-25
-
Key words:
- CT26 colon cancer cells /
- nanoliposomes /
- vaccine /
- tumor RNA
Abstract:
| Citation: | LU Guangzhao, ZHANG He, FAN Li, SUN Zhiguo, LU Ying. Effect of different injection approaches of tumor RNA nanoliposome vaccine on the growth of colon cancer[J]. Journal of Pharmaceutical Practice and Service, 2021, 39(6): 520-524, 556. doi: 10.12206/j.issn.1006-0111.202108094 |











 DownLoad:
DownLoad: