-
景天止痛膏是由肉桂、三七、当归、元胡、猪牙皂等十四味中药组成的复方制剂,具有活血化淤、消肿止痛功能,主治骨质增生、慢性腰腿痛等症。由于该制剂标准中仅包含一个当归薄层色谱鉴别,且无定量测定方法,不能全面控制制剂质量。根据全军医疗机构制剂标准提高课题要求,在原有质量标准基础上,增加了当归、川芎、三七总皂苷、元胡的薄层鉴别,同时采用高效液相色谱法,测定景天止痛膏中所含三七皂苷R1、人参皂苷 Rg1、人参皂苷 Rb1总量的含量测定方法,以此为该制剂的质量控制提供依据。
-
LC-20AD岛津高效液相色谱仪(日本岛津);紫外检测器;对照品:三七皂苷R1(批号:110745-200617)、人参皂苷Rg1(批号:111537-201204)、人参皂苷Rb1(批号:111537-201204)均购自中国食品药品检定研究院;景天止痛膏(规格:10cm×6.5cm,批号:20140201、20140301、20140401),由原南京政治学院门诊部提供;乙腈为色谱纯,水为超纯水,其他试剂均为分析纯。
-
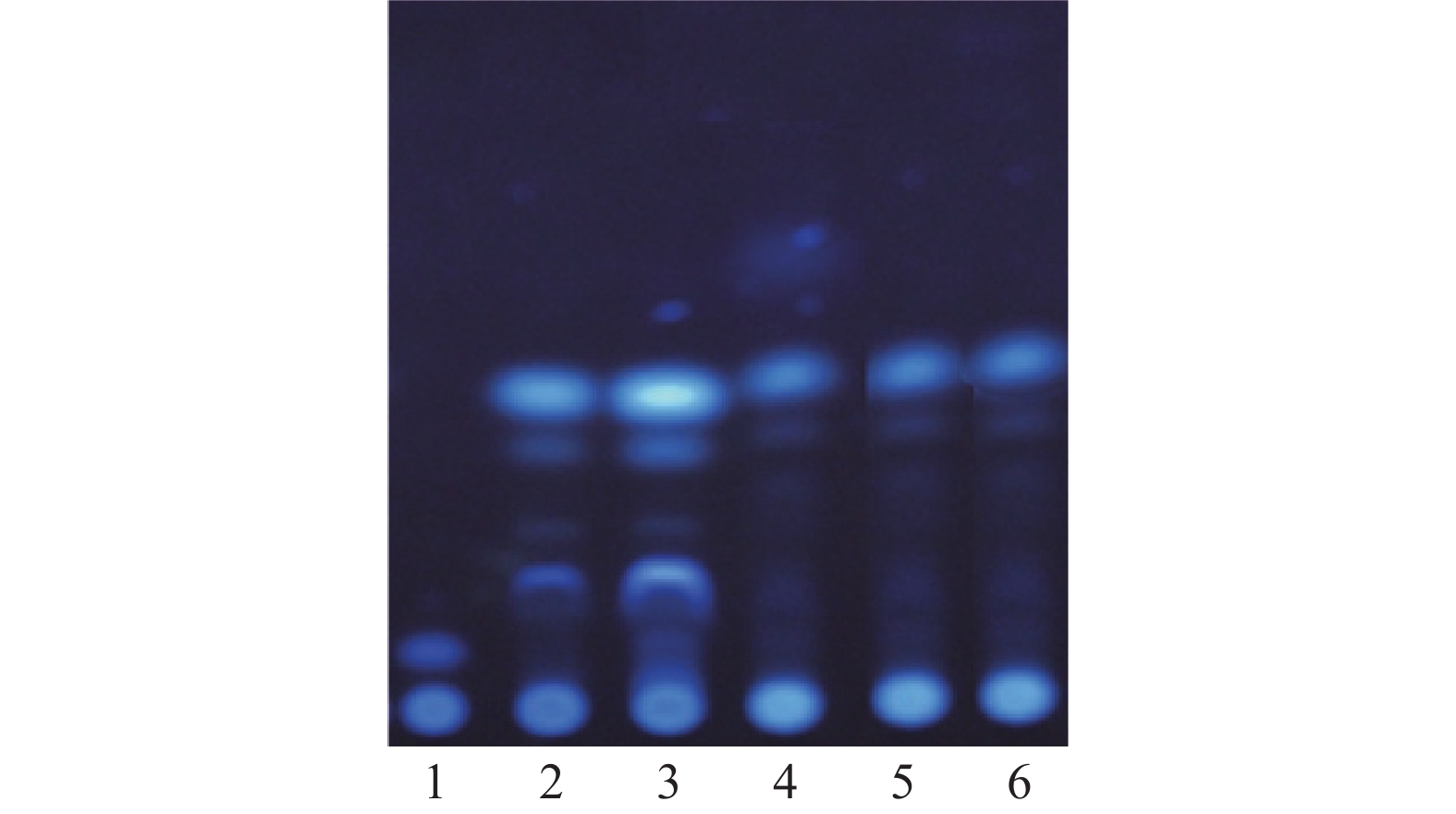
取本品1片,除去盖衬,剪成小块,置锥形瓶中,加乙醇50 ml,加热回流1 h,滤过,滤液蒸干,残渣加乙醇2 ml溶解,作为供试品溶液。另取当归、川芎对照药材适量,加乙醇分别制成每1 ml含2 mg的对照药材溶液。按照薄层色谱法(中国药典2020年版四部通则0502)试验,吸取上述两种溶液各5 μl,分别点于同一硅胶G薄层板上,以环己烷-乙酸乙酯 (9∶1)为展开剂,展开,取出,晾干,置紫外光灯(365 nm)下检视。供试品色谱中,在与对照药材色谱相应的位置上,显相同颜色的斑点。阴性试验样品未见干扰,见图1。
-
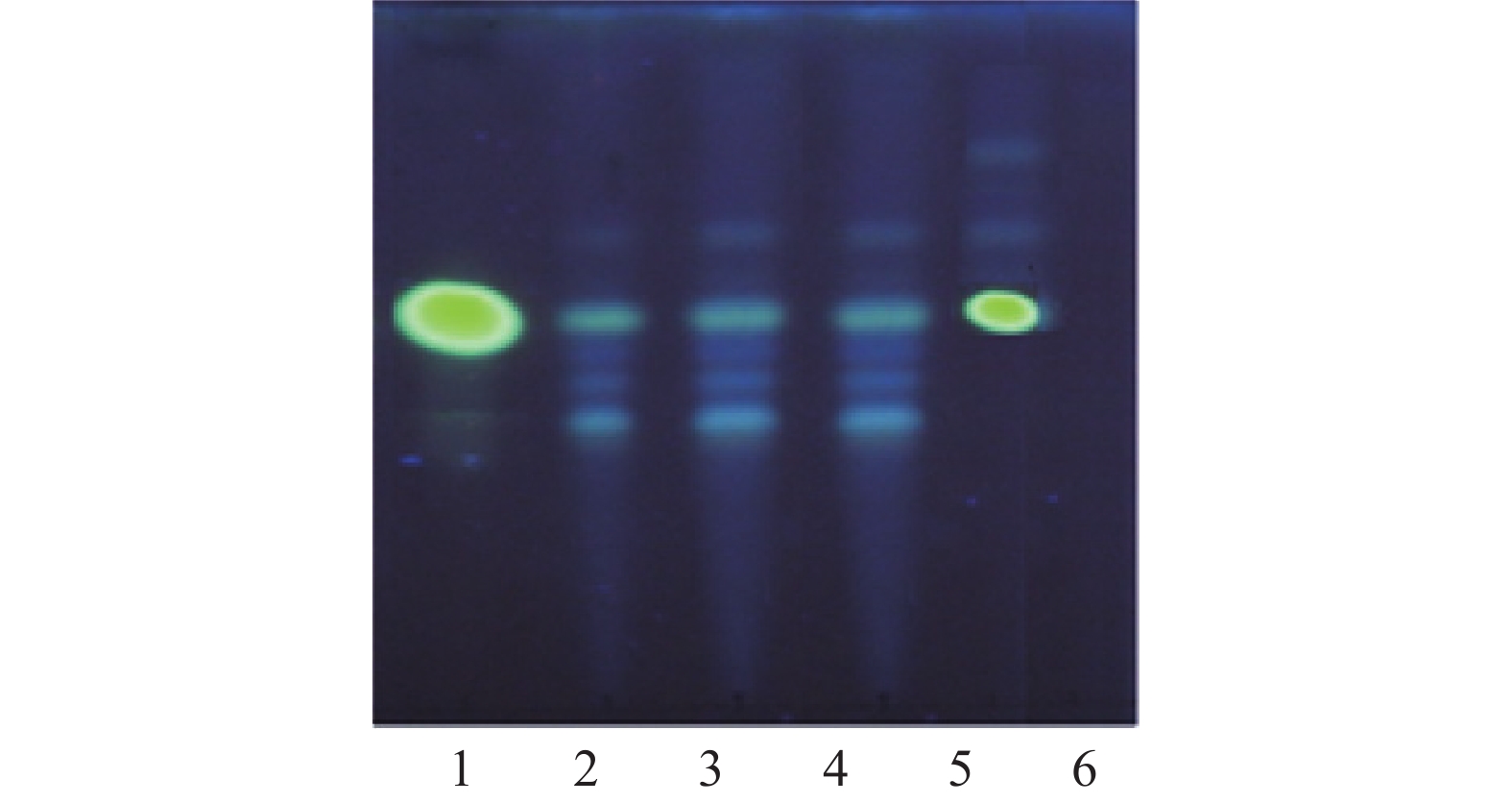
取本品2片,除去盖衬,剪成小块,置锥形瓶中,加70 %乙醇50 ml,加热回流2 h,滤过,滤液蒸干,残渣加10 ml水溶解,用1 %盐酸调pH至2~3,滤过,滤液用浓氨水调pH至9~10,用氯仿萃取3次,合并氯仿液,蒸干,残渣加甲醇1 ml使溶解。另取延胡索乙素加甲醇制成每1ml含0.5mg的对照品溶液。取元胡1 g,照供试品方法提取,加甲醇制成每1 ml含1 mg的对照药材溶液。按照薄层色谱法(中国药典2020年版四部通则0502)试验,吸取上述两种溶液各5 μl,分别点于同一硅胶G薄层板上,以乙酸乙酯-冰醋酸-水 (10∶3∶5)的上层溶液为展开剂,展开,取出,晾干,置碘缸中熏至斑点清晰后取出,挥尽板上吸附的碘后,置紫外光灯(365 nm)下检视,供试品色谱中,在与对照品色谱相应位置上显相同颜色的荧光斑点。阴性试验样品未见干扰,见图2。
-
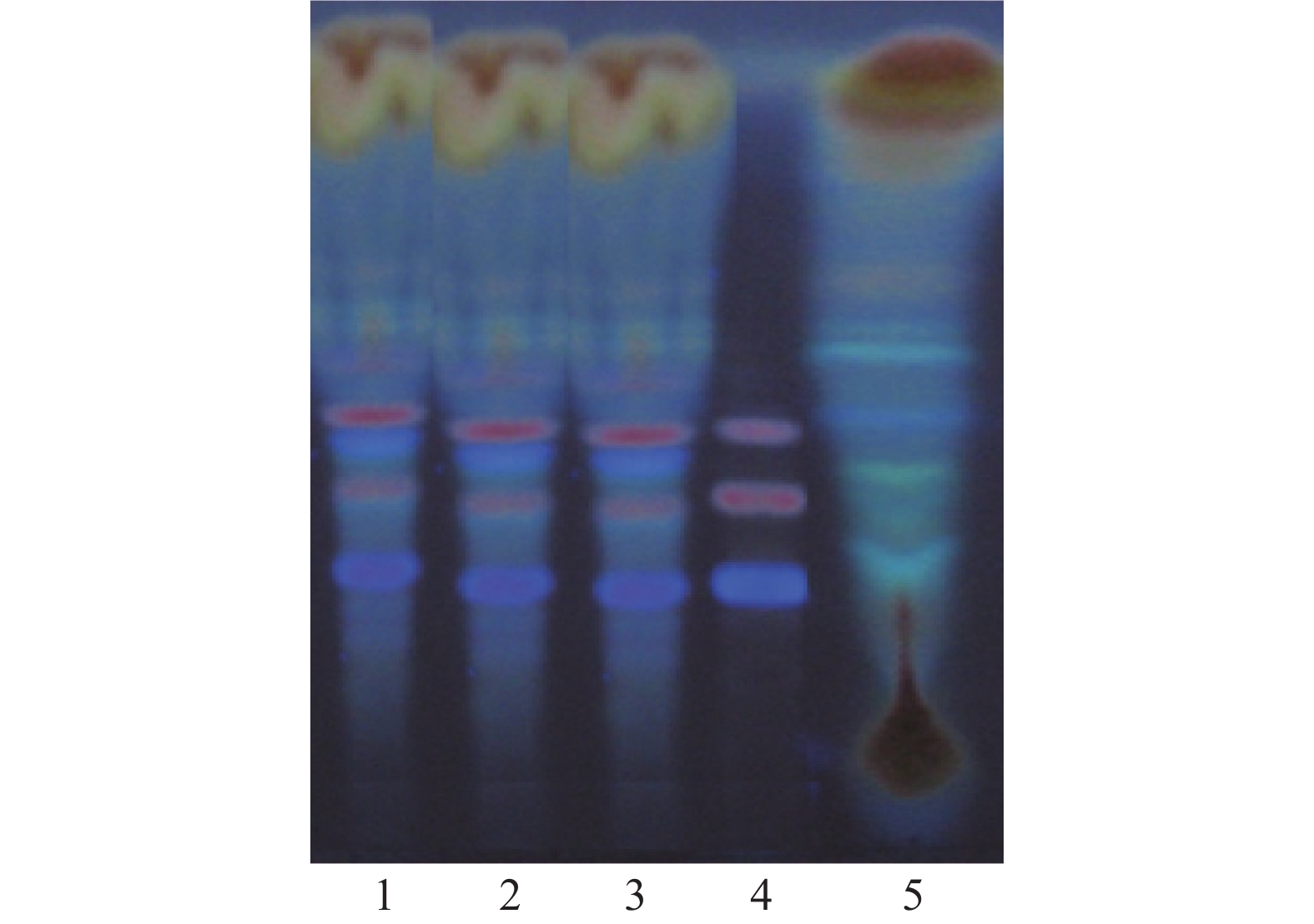
取本品2 g,除去盖衬,剪成小块,置锥形瓶中,加水5 ml,再加入水饱和后的正丁醇30 ml,振摇,静置过夜,滤过,滤液加3倍量以正丁醇饱和的水,摇匀,放置使分层,取正丁醇层,蒸干,残渣加甲醇1 ml使溶解。另取三七总皂苷,加甲醇制成每1 ml含0.5 mg的溶液。按照薄层色谱法(中国药典2020年版四部通则0502)试验,吸取上述两种溶液各5μl,分别点于同一硅胶G薄层板上,以三氯甲烷-乙酸乙酯-甲醇-水 (15∶40∶22∶10)10 ℃以下放置的下层溶液为展开剂,展开,取出,晾干,喷以10 %硫酸乙醇溶液,在105 ℃加热至斑点显色清晰。供试品色谱中,在与对照品色谱相应的位置上,显相同颜色的斑点;置紫外光灯(365nm)下检视,显相同的荧光斑点。阴性试验样品未见干扰,见图3。
-
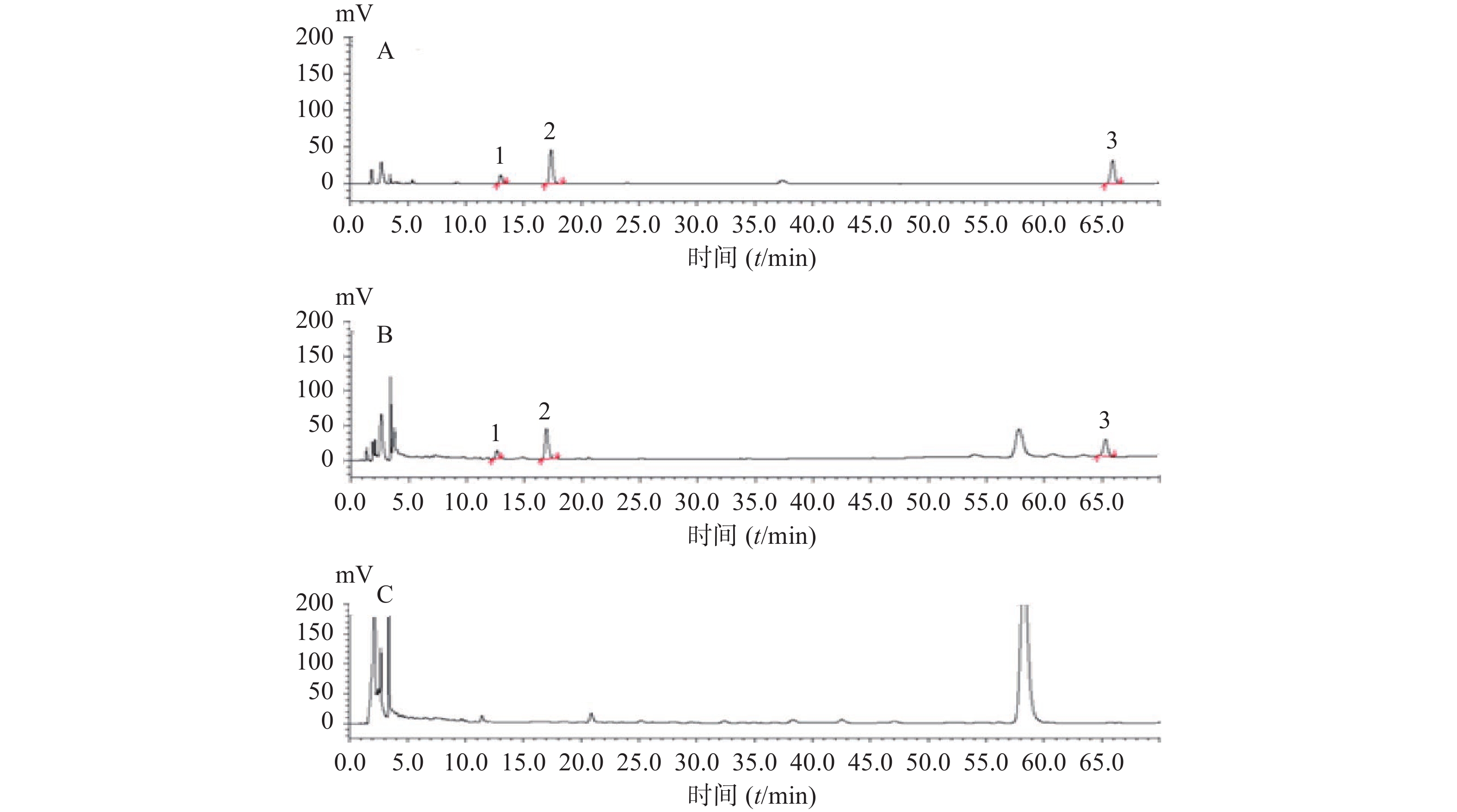
色谱柱:Promosil C18柱(250 mm×4.6 mm,5 μm);以乙腈为流动相A,水为流动相B,梯度洗脱:0~5 min,23% A;5~68 min,23%~33% A。柱温为35 ℃,流速为1.0 ml/min;检测波长为203 nm;进样体积为10 μl。在此条件下,样品中三七皂苷R1、人参皂苷Rg1、人参皂苷Rb1与相邻峰达到良好分离,见图4。
-
取三七皂苷R1、人参皂苷Rg1、人参皂苷Rb1对照品适量,精密称定,加甲醇制成每1 ml中分别含0.08、0.4、0.36 mg的混合溶液,即得。
-
取本品约2 g,除去盖衬,剪成小块,精密称定,置具塞锥形瓶中,加入甲醇50 ml,加热回流2 h,摇匀,滤过。再用15 ml甲醇荡洗锥形瓶,滤过,合并滤液并蒸干,残渣加水15 ml,以水饱和的正丁醇振摇提取4次,每次20 ml,合并正丁醇液,用氨试液洗涤2次,每次20 ml,合并正丁醇液,蒸干,残渣加甲醇溶解并转移至10 ml量瓶中,加甲醇至刻度,摇匀,滤过,取续滤液,即得。
-
按处方量配制不含三七药材的阴性样品,按供试品溶液方法制备阴性对照溶液。
-
取对照品溶液、供试品溶液、阴性对照溶液,按“2.2.1”项下色谱条件进样分析,阴性对照液中色谱峰对测定无干扰(见图4)。
-
取三七皂苷R1、人参皂苷Rg1、人参皂苷Rb1对照品适量,加甲醇溶解制成每1 ml 分别含0.8019、4.0014、3.0913 mg的混合对照品储备溶液6,将溶液6稀释如下: 0.2 ml→10 ml、0.5 ml→10 ml、1 ml→10 ml、2 ml→10 ml、2.5 ml→10 ml作为对照品溶液1~5,精密吸取上述对照品溶液1~5各10μl,注入液相色谱仪,按“2.2.1”项下色谱条件测定峰面积,以进样量(μg)为横坐标(X),峰面积(Y)为纵坐标,进行线性回归,分别得到三七皂苷R1、人参皂苷Rg1和人参皂苷Rb1的回归方程:
Y=30339X−874.4,r=0.999
Y=22431X−0.325,r=1.000
Y=27695X+699.8,r=1.000
结果表明,三七皂苷R1、人参皂苷Rg1、人参皂苷Rb1分别在0.1604~2.005 µg、0.8003~10.0035 µg和0.6182~7.7275 µg范围内呈良好的线性关系。
-
精密吸取“2.2.2”项下对照品溶液10 µl,在上述色谱条件下,连续进样6次。结果三七皂苷R1、人参皂苷Rg1、人参皂苷Rb1峰面积的RSD值依次为1.23%、1.54%、1.04%(n=6),表明仪器精密度良好。
-
取同一批号的景天止痛膏(批号20140201)按“2.2.3”项下方法平行制备6份供试品溶液,按上述色谱条件,结果三七皂苷R1、人参皂苷Rg1、人参皂苷Rb1峰面积的RSD值依次为2.86%、2.49%、2.75%,表明此法重现性良好。
-
取同一批号的景天止痛膏(批号20140201)按“2.2.3”项下方法制备供试品溶液1份,精密吸取10 µl, 分别在0、2、5、9、12、24 h进样,测定。结果三七皂苷R1、人参皂苷Rg1、人参皂苷Rb1峰面积的RSD值依次为1.85%、0.77%、1.20%,表明供试品溶液在24 h内稳定性良好。
-
取同一批号的景天止痛膏(批号20140201)1.0g,精密称定,分别精密加入混合对照品溶液(三七皂苷R10.0078 mg/ml、人参皂苷Rg10.088 mg/ml、人参皂苷Rb10.0648 mg/ml)50 ml,按“2.2.3”项下方法平行制备6份供试品溶液,按“2.2.1”项下色谱条件测定,结果见表1。
主成分 称样量(g) 样品量(mg) 加入量(mg) 测得量(mg) 回收率(%) 平均值(%) RSD(%) 三七皂苷R1 1.0130 0.3982 0.3900 0.7980 102.51 101.43 2.56 1.0676 0.4196 0.3900 0.8240 103.69 1.0210 0.4012 0.3900 0.7880 99.18 1.0590 0.4162 0.3900 0.7960 97.38 1.0004 0.3964 0.3900 0.7940 101.95 1.0002 0.3930 0.3900 0.7980 103.85 人参皂苷Rg1 1.0130 3.9770 4.4000 8.3000 98.25 98.75 2.71 1.0676 4.1914 4.4000 8.5240 98.47 1.0210 4.0084 4.4000 8.3540 98.76 1.0590 4.1576 4.4000 8.7260 103.83 1.0004 3.9276 4.4000 8.2020 97.15 1.0002 3.9268 4.4000 8.1540 96.07 人参皂苷Rb1 1.0130 2.4960 3.2400 5.7900 101.67 100.95 2.75 1.0676 2.6306 3.2400 5.9760 103.25 1.0210 2.5158 3.2400 5.8920 104.20 1.0590 2.6094 3.2400 5.8840 101.07 1.0004 2.4650 3.2400 5.6620 98.67 1.0002 2.4644 3.2400 5.6020 96.84 -
按“2.2.3”项下方法制备供试品溶液,按“2.2.1”项下色谱条件测定3批样品,结果见表2。
-
三七总皂苷为三七药材的主成分,参照2020版《中国药典》[1],以三七药材方法项下紫外吸收203 nm作为最大吸收。
-
分别取待测样品1、2、3 g,加入不同浓度乙醇、甲醇,回流1、2、3 h和冷浸24 h,采用氨试液洗涤样品等方法提取样品。结果表明,最终选择取样量为2 g,甲醇为溶剂,氨试液洗涤,并按照正文的含量测定项下方法进行测定提取较完全。
-
按照“2.2.3”项下供试品溶液的制备,分别在不同柱温30、35、40℃,不同梯度流动相比例条件(0~5 min,23 % A;5~63 min,23%~34 % A与0~5 min,23 % A;5~68 min,23%~33 % A)下,不同色谱柱InertSustainC18 (150 mm×4.6 mm, 5 μm)与Promosil C18柱(250 mm×4.6 mm,5 μm),测定三七总皂苷含量。在各试验条件下,待测成分的分离较好,测定结果基本一致,耐用性良好。
-
建立景天止痛膏制剂中当归、川芎、元胡、三七总皂苷的薄层色谱鉴别方法,拟增加肉桂的薄层实验中,因存在阴性试验样品有干扰的问题,故未列入本文。对TLC方法,分别从温度(10℃和35℃)、相对湿度(42%和88%)以及不同硅胶板(国产和进口)等方面进行考察。在各试验条件下,鉴别结果 不受温度、相对湿度和不同硅胶板等条件改变的影响。说明本文所建立的薄层色谱方法作为定性鉴定方法具有很强的适用性。
批号 三七皂苷R1
(mg/g)人参皂苷Rg1
(mg/g)人参皂苷Rb1
(mg/g)20140201 0.393 1.963 1.232 20140301 0.407 1.978 1.239 20140401 0.421 1.997 1.265 本文采用薄层色谱法鉴别了景天止痛膏制剂中当归、川芎、元胡、三七总皂苷,并建立了高效液相色谱法三七皂苷R1、人参皂苷Rg1、人参皂苷Rb1含量的测定方法。本研究结果,可为该制剂生产过程中的质量控制及检验提供参考,有助于更好把控药品疗效与临床用药安全。
Study on quality standard of Jingtian Zhitong cream
doi: 10.12206/j.issn.1006-0111.202109050
- Received Date: 2021-09-09
- Rev Recd Date: 2022-01-20
- Available Online: 2022-03-29
- Publish Date: 2022-03-25
-
Key words:
- Jingtian Zhitong cream /
- quality standard /
- HPLC /
- TLC
Abstract:
| Citation: | HU Dan, YIN Ming, WANG Xiwen, CAO Hong, ZHANG Guiying, SU Chuanyang, ZHANG Shuai. Study on quality standard of Jingtian Zhitong cream[J]. Journal of Pharmaceutical Practice and Service, 2022, 40(2): 157-160, 170. doi: 10.12206/j.issn.1006-0111.202109050 |









 DownLoad:
DownLoad: