-
近年来,有研究证实胰高血糖素样多肽-1(GLP-1)受体激动剂和钠-葡萄糖共转运蛋白-2(SGLT-2)抑制剂对2型糖尿病患者具有心血管保护作用[1-12]。中华医学会糖尿病分会第二十四次全国学术会议发布了《中国糖尿病防治指南(2020版)》,指南建议确诊动脉粥样硬化性心血管疾病(ASCVD)或伴有ASCVD高危因素的2型糖尿病患者,不论其糖化血红蛋白(HbA1c)是否达标,只要没有禁忌证都应在二甲双胍的基础上加用具有ASCVD获益证据的GLP-1受体激动剂或SGLT-2抑制剂;合并心衰(HF)的2型糖尿病患者,不论其HbA1c是否达标,只要没有禁忌证都应在二甲双胍的基础上加用SGLT-2抑制剂[13]。根据大型心血管安全性试验的结果,具有ASCVD获益的SGLT-2抑制剂和GLP-1受体激动剂有:恩格列净、卡格列净、利拉鲁肽、索马鲁肽、度拉糖肽和阿必鲁肽,其他SGLT-2抑制剂和GLP-1受体激动剂是否具有ASCVD获益还需进一步研究;具有心衰获益的仅有SGLT-2抑制剂:恩格列净、卡格列净、达格列净和艾托格列净,GLP-1受体激动剂是否具有心衰获益还需进一步研究[14]。本研究基于现有的证据进行系统回顾和网络Meta分析(NMA),评价已上市的SGLT-2抑制剂和GLP-1受体激动剂对T2DM患者心血管保护作用的疗效差异,对其进行概率排序,形成推荐等级,为我国2型糖尿病临床治疗方案的选择提供循证依据。
-
检索Medline、Embase和Cochrane Library数据库,检索日期为建库至2020年7月18日。采用主题词结合自由词的方式制定检索策略。检索词包括“diabetes mellitus, type 2” “randomized controlled trial” “empagliflozin” “canagliflozin” “dapagliflozin” “ertugliflozin” “albiglutide” “dulaglutide” “exenatide” “liraglutide” “lixisenatide” “semaglutide” “oral se-maglutide”。
-
①随机对照试验;②年龄≥18岁,2型糖尿病患者;③试验组:恩格列净、卡格列净、达格列净、艾托格列净、度拉糖肽、索马鲁肽、利拉鲁肽、利司那肽、艾塞那肽、阿必鲁肽、口服索马鲁肽;对照组:安慰剂或以上SGLT-2抑制剂、GLP-1受体激动剂中除试验组外的任意一种或多种;④干预时长≥24周;⑤结局指标:主要心血管不良事件、全因死亡、心血管死亡、心力衰竭事件。
-
使用Endnote软件进行文献管理,由2名作者独立阅读文献,按照纳入标准提取研究的一般资料、患者基本特征、结局事件等。如意见不一致,通过讨论或第3名作者介入的方式解决。
-
由2名作者根据Cochrane偏倚风险评估工具2.0独立对纳入研究进行质量评价[15],如意见不一致,通过讨论或第3名作者介入的方式解决。质量评价包括:①随机化过程产生的偏倚;②偏离既定干预措施产生的偏倚;③结局数据缺失产生的偏倚;④结局测量产生的偏倚;⑤结果选择性报告产生的偏倚;⑥整体偏倚。结果表示为“低风险”“需关注”和“高风险”,在5个领域中若出现任意一个或多个偏倚评估为高风险,则整体偏倚评估为高风险。
-
运用R软件 (version 4.0.3) 和GeMTC 包进行贝叶斯网状Meta分析,效应指标为风险比(HR)及其95%可信区间(CI)[16]。运用I2检验来评估研究间的异质性,若I2<50%,表示研究间的误差由抽样误差引起,纳入研究具有同质性;若I2>50%,认为研究间存在异质性[17]。运用节点拆分法检验直接比较结果和间接比较结果是否存在不一致性,若P>0.05,则认为直接比较和间接比较结果一致[18]。运用累积排序概率图下面积(SUCRA)作为累积排序概率的指标,根据SUCRA值的大小对干预措施优劣进行排序,SUCRA值越大表示干预措施获益越多[19]。SUCRA的值介于0到1之间。
-
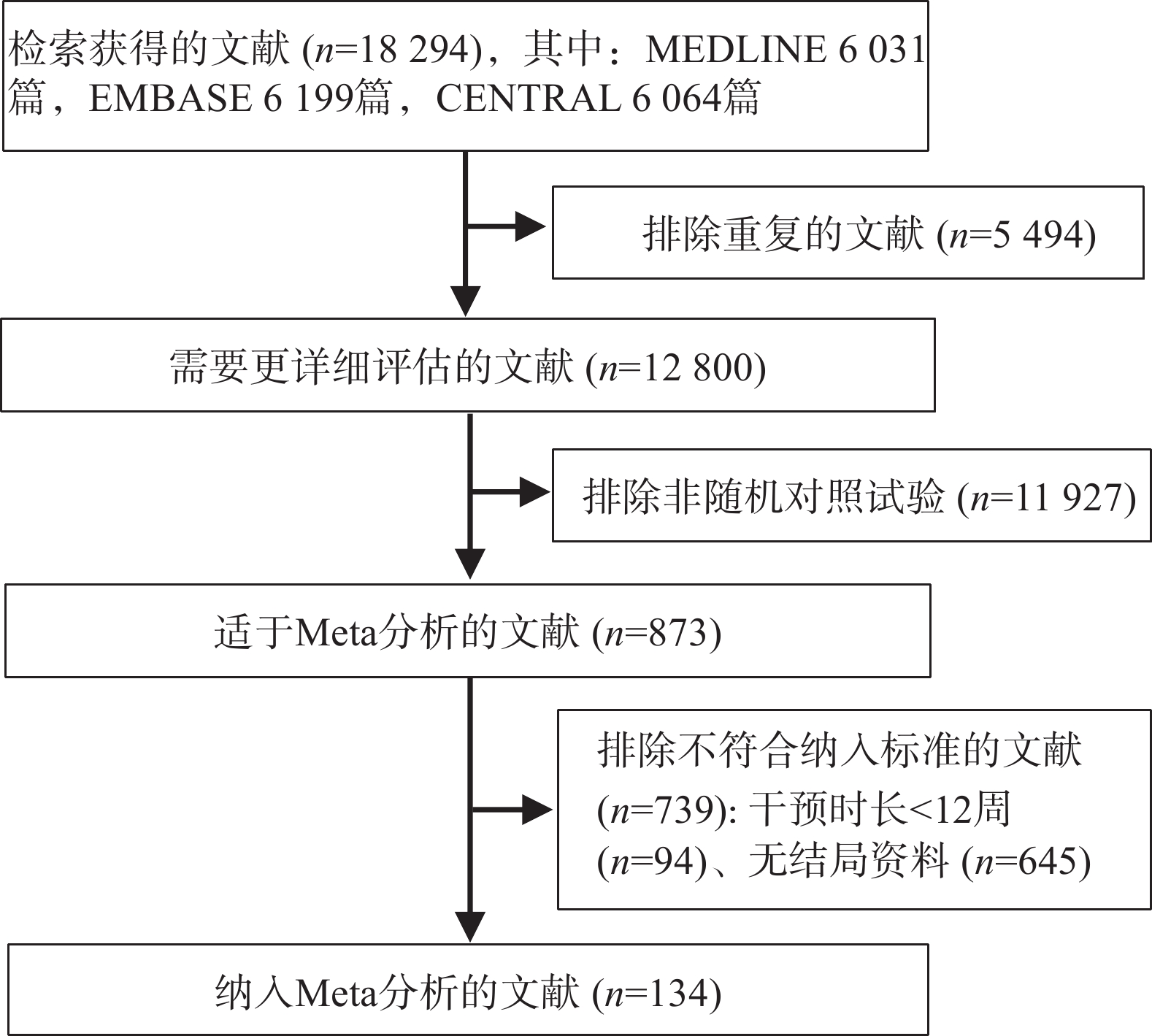
检索获得18 294篇文献,剔除5 189篇重复文献,通过阅读题目和摘要剔除11 507 篇文献,通过阅读全文剔除1 464篇文献,最后纳入134项随机对照试验(见图1),涉及11种降糖药物。
-
共纳入162 345例患者,平均年龄为58.4岁,男性占56.7%,糖尿病病史约9年,平均HbA1c为8.1%,平均空腹血糖为9.2 mmol/L,平均BMI为31.4 kg/m2,平均体重为87.8 kg,平均收缩压为132.2 mmHg,平均舒张压为78.5 mmHg。
-
整体偏倚风险评估中,66项研究为高风险,53项为低风险,14项研究偏倚风险未知。随机化过程、结局测量、结果选择性报告领域中所有研究均为低风险;在结局数据缺失的偏倚评估中,由于研究失访率大于5%、存在组间失访率不均衡,以及缺失的数据等原因,有64项研究为高风险;3项研究因未报告干预分配的分析方法,在偏离既定干预措施的偏倚评估中为高风险。
-
纳入试验的临床特征、方法学及统计学相似,结合异质性检验结果I2=0,表明纳入研究具有同质性。
-
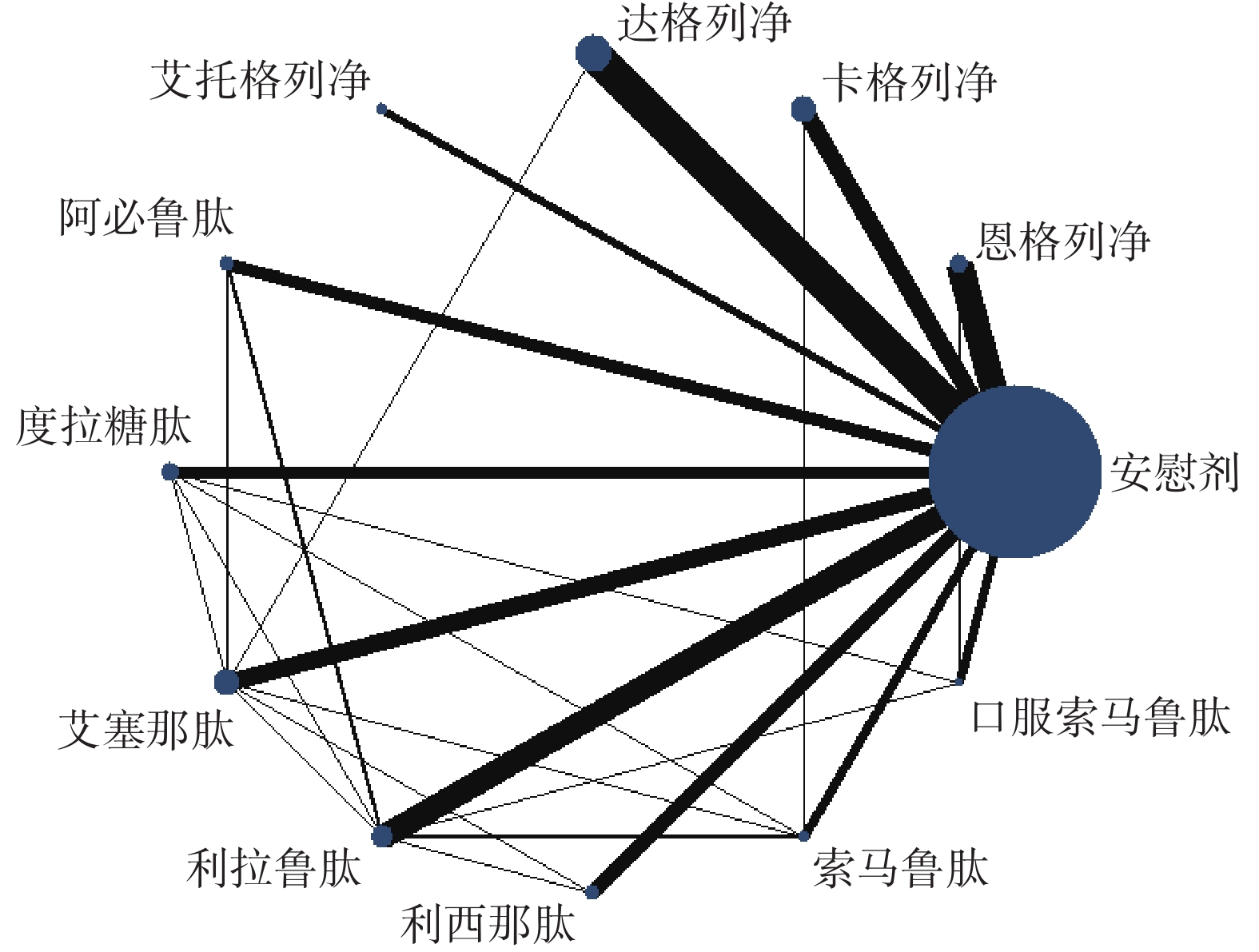
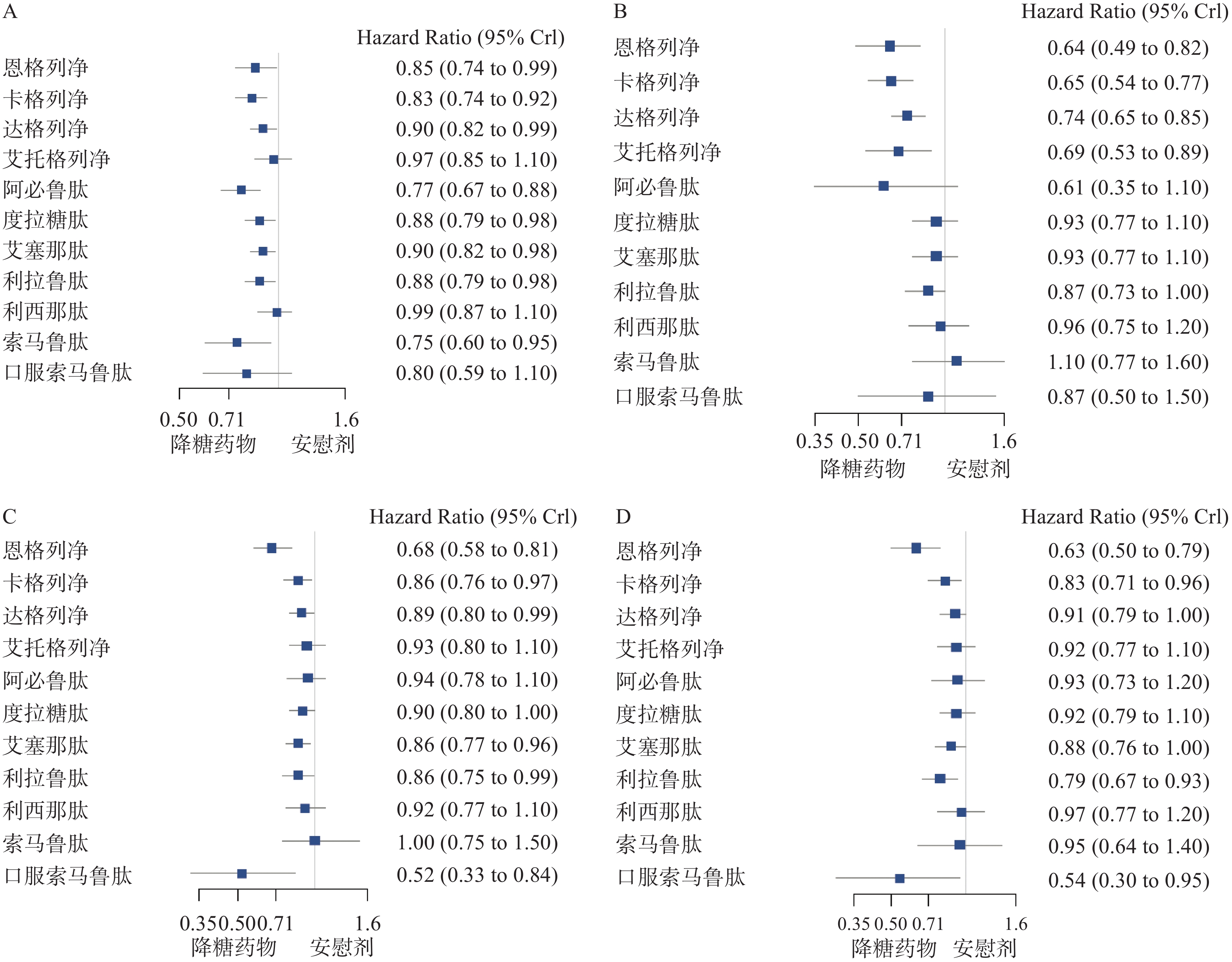
主要心血管不良事件的网状图如图2所示。与安慰剂相比,恩格列净、卡格列净、达格列净、阿必鲁肽、度拉糖肽、艾塞那肽、利拉鲁肽、索马鲁肽,可降低2型糖尿病患者主要心血管不良事件的发生风险(见图3A);与安慰剂相比,恩格列净、卡格列净、达格列净和艾托格列净均可降低2型糖尿病患者心力衰竭的发生风险(见图3B);与安慰剂相比,恩格列净、卡格列净、达格列净、艾塞那肽、利拉鲁肽和口服索马鲁肽可降低全因死亡事件的发生风险(见图3C);与安慰剂相比,恩格列净、卡格列净、利拉鲁肽、口服索马鲁肽可降低心血管死亡事件的发生风险(见图3D)。
-
纳入研究的网状Meta分析直接证据和间接证据的不一致性检验,P值均大于0.05,表明直接证据和间接证据具有一致性。
-
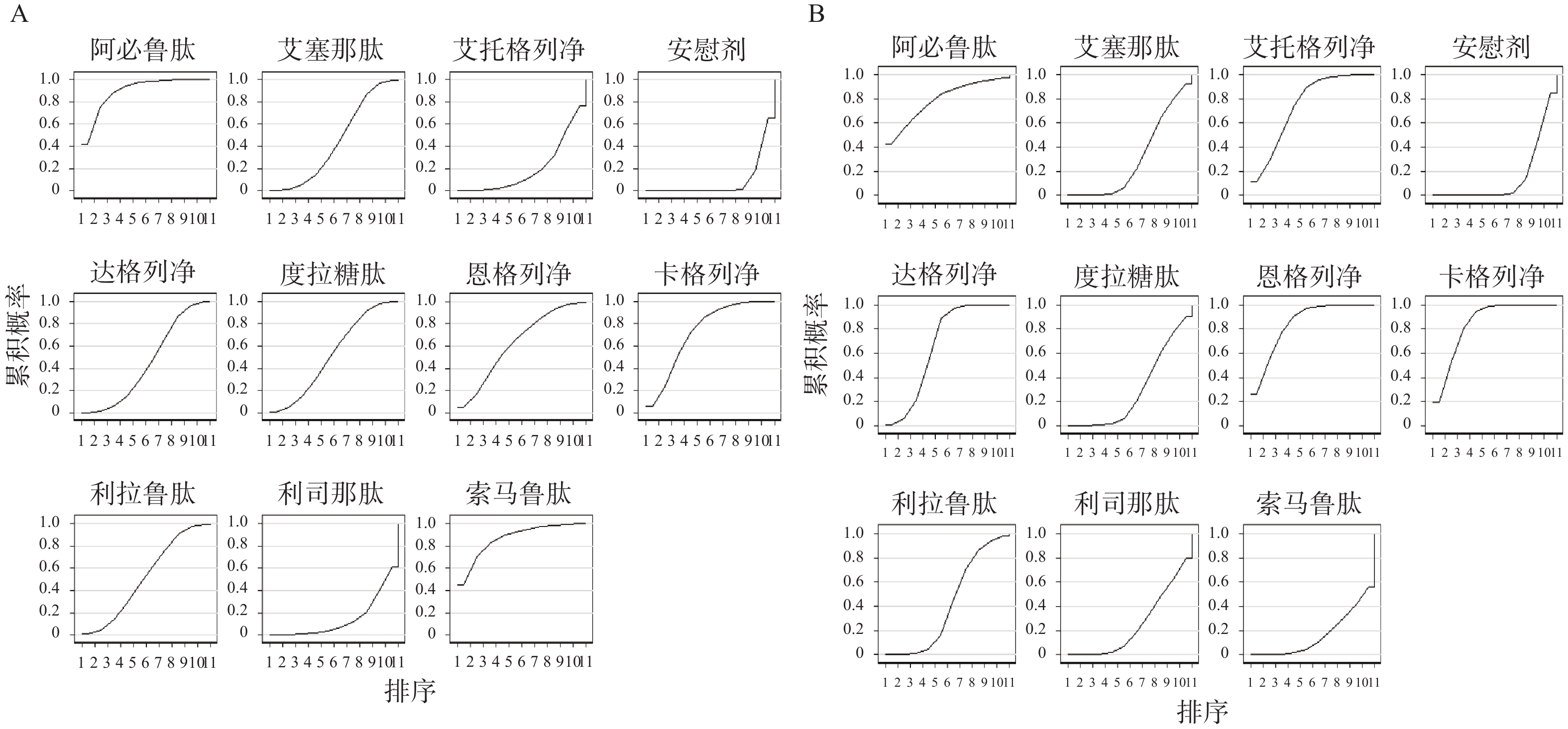
根据累积排序概率图下面积(见图4),在主要心血管事件方面,获益从高到低的排序依次是:阿必鲁肽、索马鲁肽、卡格列净、口服索马鲁肽、恩格列净、度拉糖肽、利拉鲁肽、艾塞那肽、达格列净、艾托格列净、利司那肽、安慰剂;在降低心力衰竭的发生风险方面,获益排序依次是:恩格列净、卡格列净、阿必鲁肽、艾托格列净、达格列净、口服索马鲁肽、利拉鲁肽、艾塞那肽、度拉糖肽、利司那肽、安慰剂、索马鲁肽。
-
本文研究结果显示,恩格列净、卡格列净、达格列净、阿必鲁肽、度拉糖肽、艾塞那肽、利拉鲁肽及索马鲁肽可降低2型糖尿病患者主要心血管不良事件的发生风险。结合多项大型心血管安全性试验及最新的循证医学证据,达格列净与艾塞那肽是否具有ASCVD获益还需要进一步研究,目前具有明确ASCVD获益的SGLT-2抑制剂和GLP-1受体激动剂有恩格列净、卡格列净、阿必鲁肽、度拉糖肽、利拉鲁肽和索马鲁肽。在我国可供选择的具有明确ASCVD获益的SGLT-2抑制剂和GLP-1受体激动剂有恩格列净、卡格列净、度拉糖肽、利拉鲁肽。结合本文研究结果,2型糖尿病合并ASCVD患者,应用SGLT-2抑制剂或GLP-1受体激动剂心血管获益从高到低分别是:卡格列净、恩格列净、度拉糖肽、利拉鲁肽。
-
本研究结果显示,恩格列净、卡格列净、达格列净和艾托格列净,均可降低2型糖尿病患者心力衰竭的发生风险,CVOTs及其他循证医学研究与本文研究结果一致,同时4种SGLT-2抑制剂均已进入我国协议期谈判药品目录。结合本文研究结果,降低心力衰竭风险方面,2型糖尿病合并心衰的患者应用SGLT-2抑制剂或GLP-1受体激动剂获益从高到低依次是:恩格列净、卡格列净、艾托格列净和达格列净。
-
前期通过系统检索数据库后发现我国上市的贝那鲁肽、洛塞那肽及tofogliflozin、ipragliflozin等在其他国家上市的SGLT-2抑制剂或GLP-1受体激动剂的随机对照试验较少,报告心血管事件的研究接近于0,因此本研究在制定纳排标准时仅纳入了11种SGLT-2抑制剂和GLP-1受体激动剂。经过筛选后纳入的134篇文献,由于试验失访率高于5%、失访数据存在组间不平衡及失访数据可能影响结果稳定性等原因,在评估偏倚风险时有64项研究在结局数据缺失的偏倚评估中为高风险,为检验研究结果的稳定性,本文通过敏感性分析纳入偏倚风险低的随机对照试验,其结果与主要研究一致。
-
根据本文的研究结果及目前在我国上市的SGLT-2抑制剂或GLP-1受体激动剂,针对2型糖尿病合并ASCVD患者,心血管获益从高到低分别是:卡格列净、恩格列净、度拉糖肽、利拉鲁肽;针对2型糖尿病合并心衰 的患者,应用SGLT-2抑制剂或GLP-1受体激动剂获益从高到低依次是:恩格列净、卡格列净、艾托格列净、达格列净。
Cardiovascular benefits of SGLT-2 inhibitors and GLP-1 receptor agonists in type 2 diabetes: a systematic review and network meta-analysis
doi: 10.12206/j.issn.1006-0111.202109108
- Received Date: 2021-09-23
- Rev Recd Date: 2021-12-30
- Available Online: 2022-07-27
- Publish Date: 2022-07-25
-
Key words:
- SGLT-2 inhibitors /
- GLP-1 receptor agonists /
- type 2 diabetes /
- cardiovascular benefits /
- network meta-analysis
Abstract:
| Citation: | LAI Yanlan, HUANG Aiwen, CHEN Guanxu, CHEN Tingting, ZHAO Lijun, LIAO Xiaolan, GUO Xiuqiang, WU Gang, SONG Hongtao. Cardiovascular benefits of SGLT-2 inhibitors and GLP-1 receptor agonists in type 2 diabetes: a systematic review and network meta-analysis[J]. Journal of Pharmaceutical Practice and Service, 2022, 40(4): 354-358. doi: 10.12206/j.issn.1006-0111.202109108 |








 DownLoad:
DownLoad: