-
白念珠菌是一种条件致病真菌,在健康人体口腔或胃肠道中以无害共生菌的形式存在。在免疫功能低下的患者中,能够导致危及生命的全身性感染。近年来,随着癌症患者、器官移植接受者等免疫功能低下人群不断增加,白念珠菌感染引发的严重疾病的发病率不断攀升[1-2],死亡率粗略估计已高于40%[3]。
高适应性是白念珠菌传播和致病的关键因素之一,包括对宿主和外界环境的适应性、形态的转换以及对抗真菌药物的适应性。代谢的改变能够影响白念珠菌的药物敏感性,Ene等[4]发现培养基中碳源由葡萄糖转变为乳酸后白念珠菌对抗真菌药物(咪康唑、两性霉素B、卡泊芬净)的敏感性发生改变。线粒体作为重要的代谢细胞器, 其中的某些基因缺失或者功能缺陷,会导致白念珠菌药物敏感性的变化,Sun等[5]发现线粒体复合体I相关基因GOA1和NDH51的缺失会导致白念珠菌对唑类药物敏感性上升,Edwina等[6]的研究发现线粒体关键基因FZO1的缺失能够增强白念珠菌对唑类药物的敏感性。
本课题组前期发现SDH2基因缺失导致白念珠菌致病力显著下降,并发现白念珠菌无法利用非发酵碳源[7]。生物信息学分析显示SDH2基因编码琥珀酸脱氢酶(SDH)的铁硫亚基,琥珀酸脱氢酶在三羧酸循环和线粒体电子传递链中均发挥作用,是能量代谢中重要的一环。SDH2基因可能在白念珠菌代谢过程中发挥重要的作用,那么它是否能够通过影响代谢从而改变白念珠菌的环境适应性呢?本研究聚焦SDH2基因对白念珠菌环境适应性的影响,包括对外界压力应答和药物敏感性的影响,并探索其可能的机制。
-
挑取少量冻存于−80℃、30% 甘油中的菌株,在YPD 液体培养基中活化,30℃,200 r/min振荡培养24 h后,吸取10 μl置于新的1 ml YPD液体培养基中,继续在 30℃,200 r/min振荡条件下培养 16 h,用 SDA 固体培养基划板,30℃培养 48 h,置于 4℃ 保存备用。实验时挑取 SDA 平板上的单克隆菌落置于1 ml YPD 液体培养基中,30℃,200 r/min振荡培养 16 h 过夜,使其处于对数生长期用于后续实验。
-
白念珠菌活化16 h ,用YPD液体培养基调整菌浓度至3×106细胞/ml。制备每种菌株的系列稀释液,以10倍倍比稀释成5个浓度梯度,每种白念珠菌的系列浓度稀释物各取5 μl,点在事先加入了待考察的不同浓度的化学试剂和药物的YPD琼脂平板上,倒置于孵箱内,25、30、37 ℃培养24~48 h,期间观察各菌株之间生长的差异并拍照记录。
-
收集处于对数生长期的白念珠菌细胞悬浮液,用无菌PBS洗涤3次。随后将细胞重悬于无菌PBS中调整其浓度至5×107细胞/ml,孵育2 h以耗尽能量,并加入终浓度为10 μmol/L的罗丹明6G。30℃、200 r/min振荡培养细胞悬浮液60 min,使罗丹明6G积累。洗涤白念珠菌细胞悬浮液3次,注意要保证白念珠菌细胞最终浓度为5×107细胞/ml。然后加入终浓度为20 mmol/L的葡萄糖。分别在25 、30、37℃ 条件下,200 r/min振荡培养白念珠菌细胞悬浮液2 h,取白念珠菌细胞样品(约1 ml)离心,收集上清液,在527 nm处测量吸光度。
-
使用GraphPad Prism软件对全部数据进行分析,时序检验 (Mantel Cox) 方法比较各组差异 ,当P<0.05时,表示差异具有统计学意义。
-
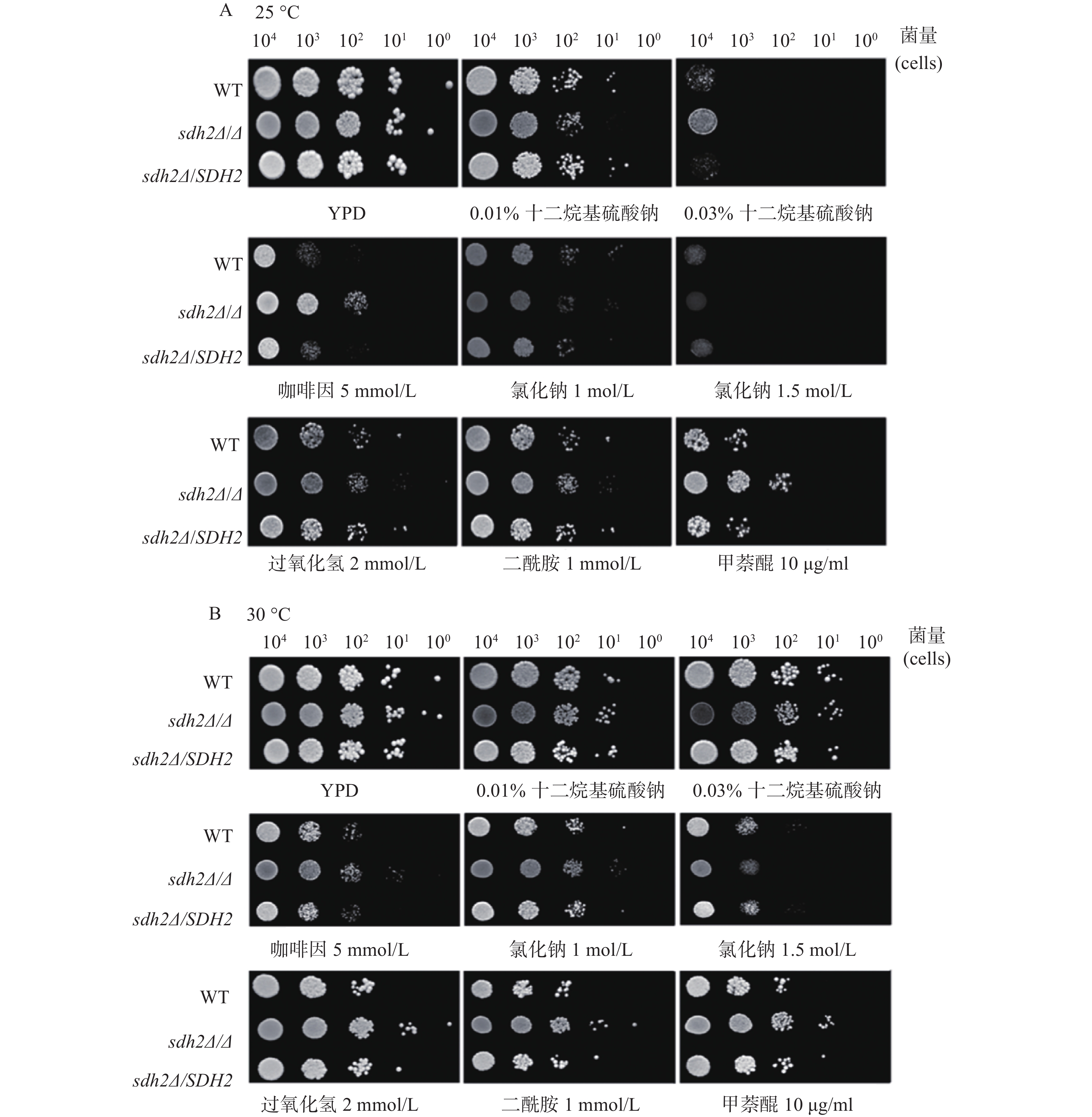
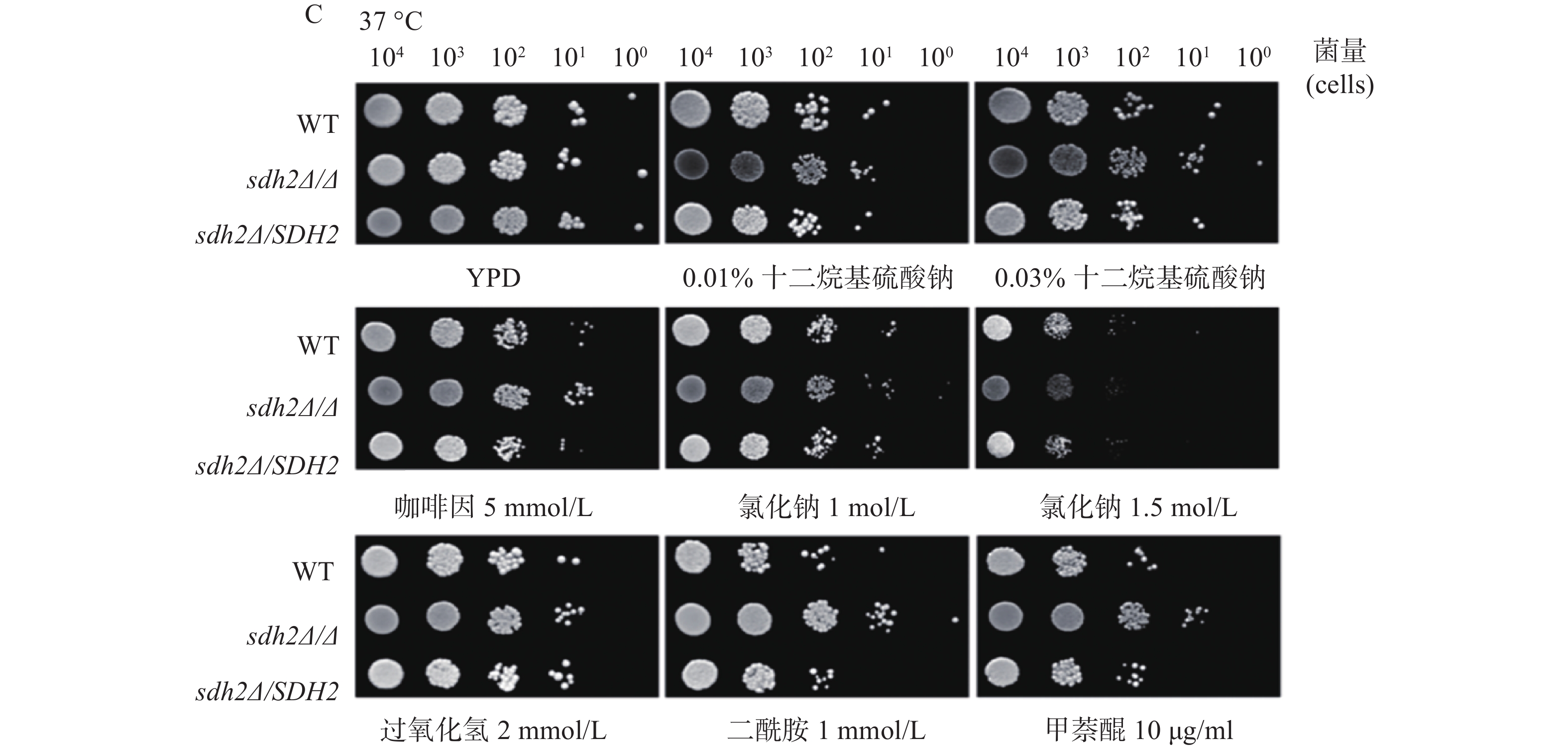
我们利用多种刺激剂考察了SDH2在白念珠菌应激反应中的作用。实验应用了sdh2Δ/Δ敲除菌、野生型菌和回复菌,考察在存在应激刺激剂的培养基中sdh2Δ/Δ敲除菌的生长情况与野生型菌和回复菌的区别,从而判断SDH2在白念珠菌应激反应中的作用。应激刺激剂包括细胞壁应激刺激剂(十二烷基硫酸钠和咖啡因)、渗透压应激刺激剂(氯化钠)、氧化应激刺激剂(过氧化氢、二酰胺和甲萘醌)。敏感性实验在25、30、37 ℃三种不同的温度下进行,将不同浓度相同体积的白念珠菌接种于琼脂培养板上,同样的培养板制备3份,白念珠菌生长形成明显的菌落后拍照。结果显示,与野生型菌或回复菌相比,sdh2Δ/Δ敲除菌于25 ℃在0.03%十二烷基硫酸钠培养板上生长得多(图1A)。此外,在所有培养温度下,sdh2Δ/Δ敲除菌在含咖啡因、二酰胺或甲萘醌的培养板上也较野生型菌/回复菌生长得多 (图1A、1B、1C)。
-
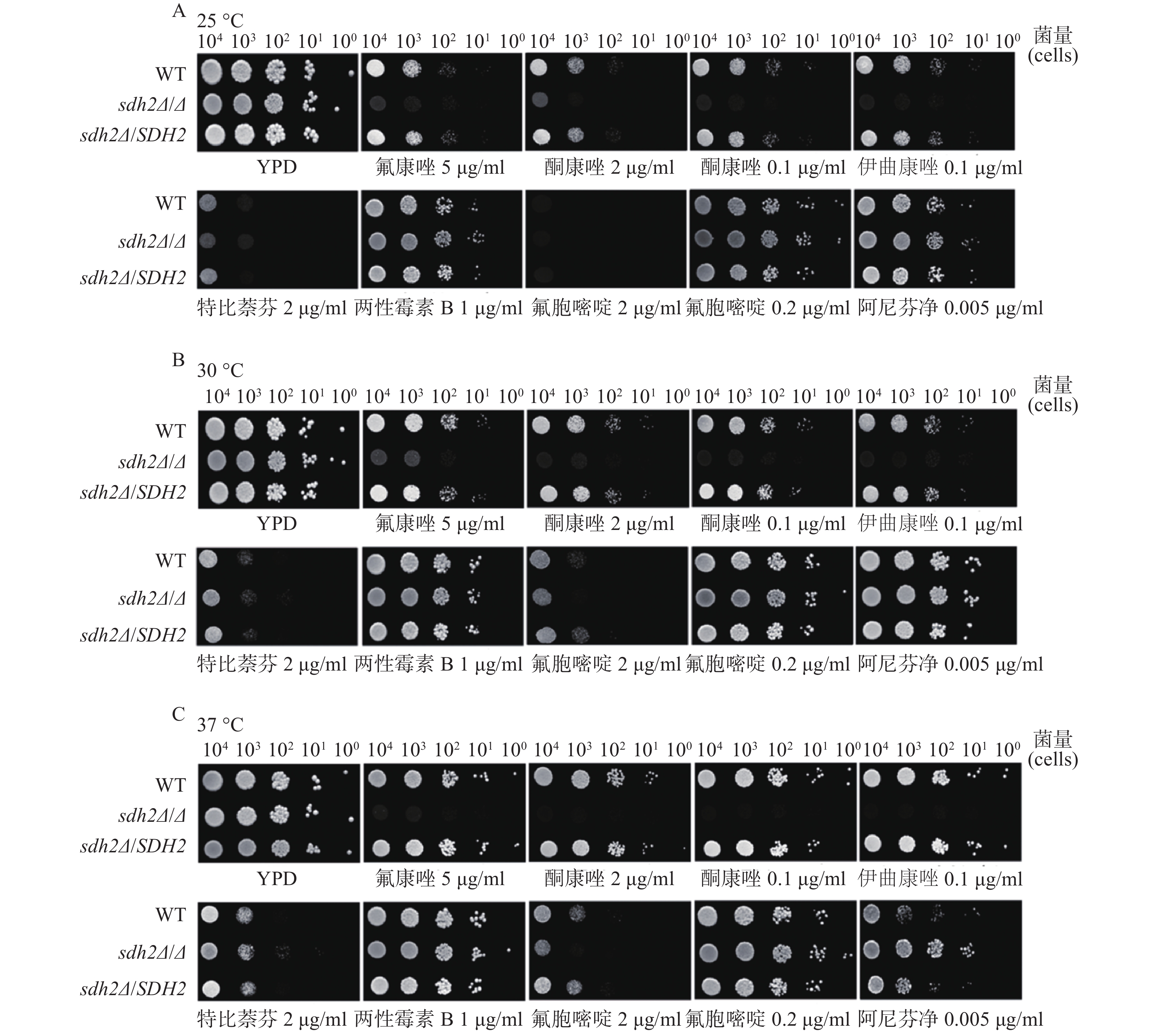
我们考察了sdh2Δ/Δ敲除菌、野生型菌和回复菌对抗真菌药物的敏感性,考察的药物包括唑类抗真菌药物特比萘芬、两性霉素B、氟胞嘧啶和阿尼芬净。将不同浓度相同体积的白念珠菌接种于琼脂培养板上,同样的培养板制备3份,白念珠菌生长形成明显的菌落后拍照。药物敏感性实验结果显示,与野生型菌/回复菌相比,sdh2Δ/Δ敲除菌在含有氟康唑、酮康唑和伊曲康唑的培养板上生长得明显减少(图2A、2B、2C),而在含有其他抗真菌药物,如特比萘芬、两性霉素B、氟胞嘧啶和阿尼芬净的培养板上没有明显差别。
-
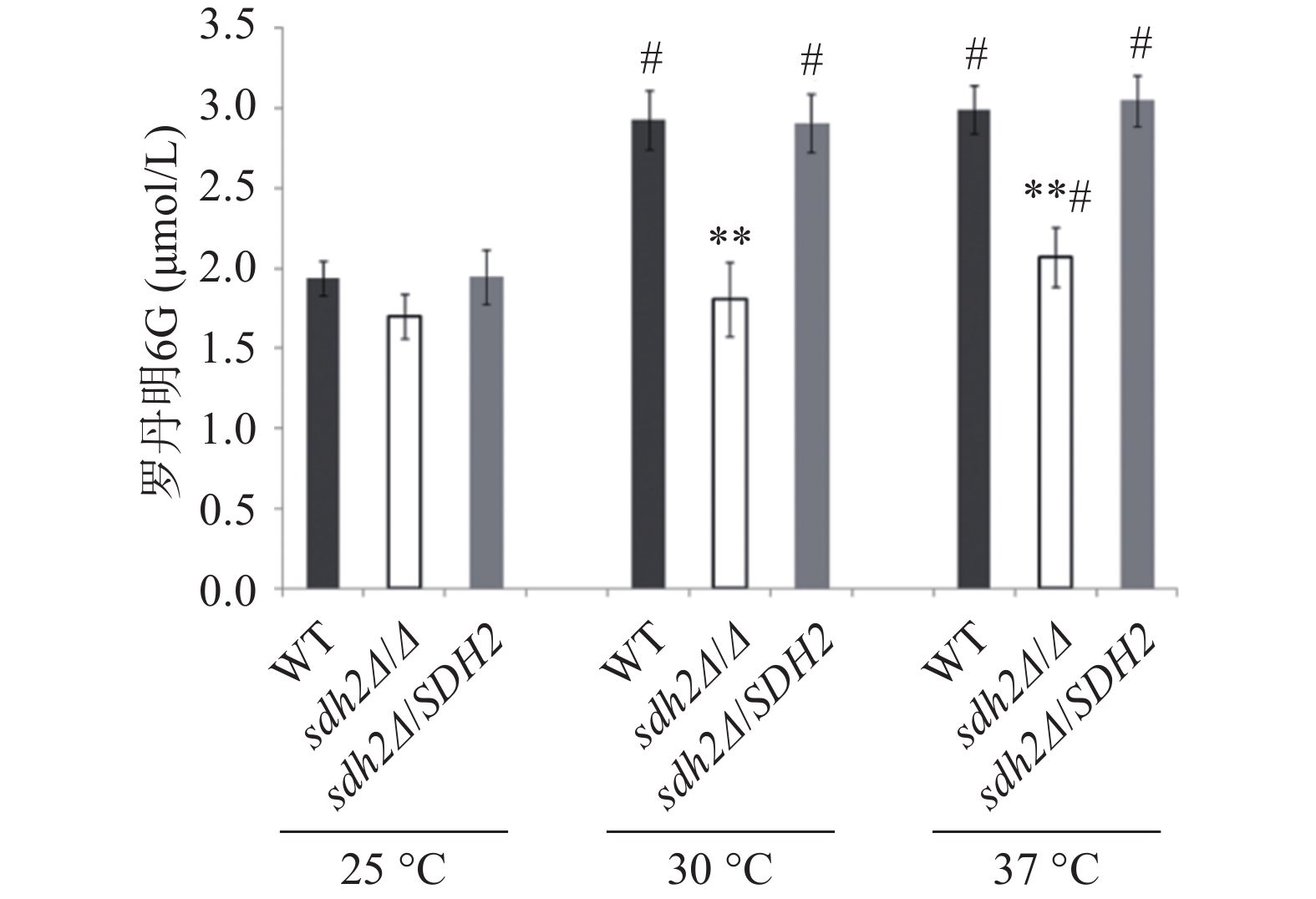
罗丹明6G是ABC(ATP-binding cassette)转运蛋白的特异性底物,在能量不足时以被动扩散的方式进入白念珠菌细胞,加入葡萄糖供能后,可通过药物外排泵以主动转运的方式泵出细胞外。我们通过罗丹明6G的外排实验考察了SDH2敲除对白念珠菌药物外排能力的影响。结果显示在30 ℃和37 ℃ 培养温度下,通过添加葡萄糖以启动能量代谢2 h后,与野生型菌相比,从sdh2Δ/Δ敲除菌的细胞中泵出的罗丹明6G显著减少(P<0.01,见图3)。有趣的是,在25 ℃ 的培养温度下,3种菌株泵出的罗丹明6G都较30 ℃和37 ℃条件下有所减少,但各菌株之间没有显著差异(图3)。
-
SDH2编码琥珀酸脱氢酶中的一个亚基,琥珀酸脱氢酶参与三羧酸循环,并且它位于线粒体内膜上,发挥电子传递的作用,对于能量代谢十分重要[8]。我们对SDH2基因在白念珠菌环境适应性中的作用进行了初步探索,实验选择在30 ℃(白念珠菌最适宜生长温度)、37 ℃(宿主温度)和25 ℃(室温)三种温度下进行。实验之所以选用三种温度,一方面是由于在不同环境温度下白念珠菌的代谢可能不同,选用不同的温度可以考察温度造成的差别;另一方面在三种温度下考察SDH2基因的功能,结果相互之间可以比较或者印证,增加信息量,有利于分析SDH2基因的功能。有意思的是SDH2基因缺失后菌株对唑类抗真菌药物(氟康唑、酮康唑、伊曲康唑)的敏感性显著上升,而对于其他类型的抗真菌药物包括丙烯胺类特比萘芬、多烯类两性霉素B、核苷类氟胞嘧啶和棘白菌素类阿尼芬净,其敏感性和野生型菌没有差异。唑类药物的敏感性与药物外排泵的功能密切相关,药物外排功能障碍可导致对唑类抗真菌药物敏感性增加[9]。药物外排泵包括ABC转运蛋白超家族和主要易化因子超家族,其中ABC转运蛋白中的Cdr1p、Cdr2p发挥作用都需要消耗能量。罗丹明6G是能量依赖型ABC转运蛋白的特异性底物,通过外排泵以能量依赖的主动转运方式排出细胞外。罗丹明6G外排实验的结果能显示能量依赖型ABC转运蛋白的功能。研究发现SDH2缺失后白念珠菌在30 ℃和37 ℃时外排能力都显著降低。SDH2对能量代谢非常重要,SDH2基因缺失后,菌株能量代谢异常,能量依赖型ABC转运蛋白的功能下降,缺失菌对唑类抗真菌药物的敏感性增加。
SDH2基因缺失后白念珠菌对咖啡因、二酰胺和甲萘醌都表现出轻微的耐受,这提示SDH2基因在白念珠菌环境应激中有一定的作用,这些环境因素包括细胞壁压力的刺激以及氧化刺激,具体的作用机制还有待进一步探讨。
综上所述,本研究发现SDH2基因缺失会导致白念珠菌对环境压力应激反应的改变;此外,SDH2基因缺失后菌株的药物外排能力降低,菌株对唑类抗真菌药物的敏感性增加。代谢对白念珠菌的环境适应能力和致病力十分重要,SDH2基因缺失导致白念珠菌对唑类抗真菌药物专特性地敏感,以SDH2为靶基因,开发真菌特异性SDH2抑制剂,有望发现与唑类药物协同的新型抗真菌药物。
The role of SDH2 gene in the environmental adaptability of Candida albicans
doi: 10.12206/j.issn.1006-0111.202201096
- Received Date: 2022-01-26
- Rev Recd Date: 2022-06-23
- Available Online: 2022-07-27
- Publish Date: 2022-07-25
-
Key words:
- Candida albicans /
- SDH2 /
- environmental adaptability /
- stress response /
- drug sensitivity
Abstract:
| Citation: | WANG Xinrong, LU Renyi, WANG Yan. The role of SDH2 gene in the environmental adaptability of Candida albicans[J]. Journal of Pharmaceutical Practice and Service, 2022, 40(4): 309-313. doi: 10.12206/j.issn.1006-0111.202201096 |








 DownLoad:
DownLoad: