-
近红外光响应的肿瘤光热治疗因具有组织穿透性强、生物安全性好等优点,已引起广泛关注。作为新兴的光敏剂,八丁氧基酞菁钯具有成本低、易合成、毒性低等优点,在肿瘤光动力治疗(PDT)领域表现出巨大潜力。由于高渗透强滞留效应(EPR),脂质体作为药物载体可靶向集中于肿瘤组织,同时ROS可氧化破坏脂质双层膜,促进包载药物在肿瘤组织的精准释放。本实验以雷帕霉素为模型药物,采用课题组合成的八丁氧基酞菁钯为光敏剂,制备近红外光触发的ROS响应型雷帕霉素脂质体,并对其理化性质及体外释放特性进行考察。
-
R206D旋转蒸发仪(上海申生科技有限公司);730 nm/1500 mW激光器(长春市亮丽光电有限公司);U3000高效液相色谱仪(Thermo Fisher公司);JY92.IIDN超声波细胞粉碎机(宁波新芝生物科技股份有限公司);马尔文激光粒度仪(英国Malvern公司)。
-
二硬脂酰磷脂酰胆碱(DSPC,Avanti公司);1,2-二亚油酰基-sn-甘油基-3-磷酸胆碱(DLPC,Avanti公司);二硬脂酰基磷脂酰乙醇胺-聚乙二醇2000(DSPE-mPEG2000,Lipoid 公司);胆固醇、雷帕霉素(MCE公司)、八丁氧基酞菁钯[PdPC(OBu)8,实验室自制];磷酸盐缓冲液(PBS,HyClone 公司)。
-
采用薄膜分散法制备脂质体。精密称取DSPC、DLPC、胆固醇、DSPE-mPEG2000、雷帕霉素、八丁氧基酞菁钯置于250 ml圆底烧瓶中,加入适量氯仿溶解。65 ℃水浴,65 r/min减压旋转蒸发形成均匀薄膜,取PBS溶液4 ml水化。吸出脂质体,用PBS稀释至5 ml,200 W探头超声5 min,即得近红外光触发ROS响应型雷帕霉素脂质体。空白脂质体除不加雷帕霉素、八丁氧基酞菁钯外,制备方法相同。
-
以脂质体成膜及水化效果、药物包封率及载药量、包载药物光敏释放效率为指标,从DLPC、胆固醇、DSPE-mPEG2000处方量及PdPC(OBu)8投药量对光敏ROS脂质体处方进行单因素考察。
-
取少量脂质体溶液,用去离子水稀释,使用马尔文激光粒度仪测量脂质体粒径、PDI及Zeta电位。
-
色谱柱:Agilent TC-C18(2)(4.6 mm×250 mm,5 μm);流动相:甲醇-水(84∶16);流速1.0 ml/min;检测波长:278 nm;柱温:50 ℃;进样量:20 μl。
-
精密称取雷帕霉素2.50 mg置于10 ml容量瓶中,用甲醇定容至刻度,得到浓度为250 μg/ml储备液。精密吸取储备液800、400、200、100、40、20、10、4 μl,分别于5 ml容量瓶中用甲醇定容。制成浓度为40、20、10、5、2、1、0.5、0.2 μg/ml的对照品溶液。HPLC仪检测并记录峰面积A,绘制出峰面积A与对照品质量浓度C的标准曲线并进行线性回归分析。
-
吸取雷帕霉素脂质体溶液500 μl加入5 ml容量瓶中,甲醇定容至刻度。超声20 min破乳后,以10 000 r/min进行超速离心10 min。取适量上清液过0.45 μm有机膜,制得供试品溶液。吸取空白脂质体按同法制备阴性对照品溶液。分别取对照品、阴性对照品、供试品按照“1.6.1”项下方法进样,记录HPLC图。
-
选取低、中、高3个浓度的对照品溶液,1 d内重复进样考察日内精密度,连续3 d每天测定一次,考察日间精密度。
-
吸取用于制备选定的低、中、高3个浓度对照品溶液的储备液于不同的5 ml容量瓶中,同一浓度共制备3个样品。向每个容量瓶中加入100 μl空白脂质体溶液,再用甲醇定容至刻度。超声20 min破乳后,进行超速离心,10 000 r/min,10 min。取适量上清液过0.45 μm有机膜,进行HPLC检测。
-
包封率与载药量是评价脂质体的重要指标。包封率是脂质体中包封药物量与脂质体中药物总量的百分比。载药量是脂质体中药物量与脂质体总量的百分比。
精密吸取脂质体500 μl置于5 ml容量瓶中,甲醇稀释至刻度。超声20 min破乳后,再进行超速离心,10 000 r/min,10 min。取适量上清液过0.45 μm有机膜,进行HPLC检测,计算得出脂质体中药物总量。
精密吸取适量脂质体溶液,先进行低速离心[3],3 000 r/min,15 min。取上层液体500 μl置于5 ml容量瓶中用甲醇稀释至刻度。超声20 min破乳后,再进行超速离心,10 000 r/min,10 min。取适量上清液过0.45 μm有机膜,进行HPLC检测,计算出脂质体中包封药物量。
-
采用反向透析法测定雷帕霉素脂质体光敏释放特性。以30 ml 20%乙醇为释放介质,加入50 ml离心管内;另吸取1 ml释放介质并加入透析袋内,将透析袋两端扎紧后放入离心管中;取300 μl雷帕霉素脂质体,用730 nm,1 W/cm2近红外光照射5 min后,转移至释放介质;将离心管移至摇床内,设置摇床条件为37 ℃,180 r/min。分别在1、2、4、8、12 h从透析袋内取样200 μl,用于样品测定和累积释放率的计算。取样后即补加新鲜释放介质200 μl,保持释放介质总量不变。以时间(t)为横坐标,以累积释放率(Q%)为纵坐标,绘制释放曲线。
-
回归方程为A = 0.6240 C-0.1738 (r = 0.999 5)。雷帕霉素在0.2~40 μg/ml浓度范围内线性关系良好。
-
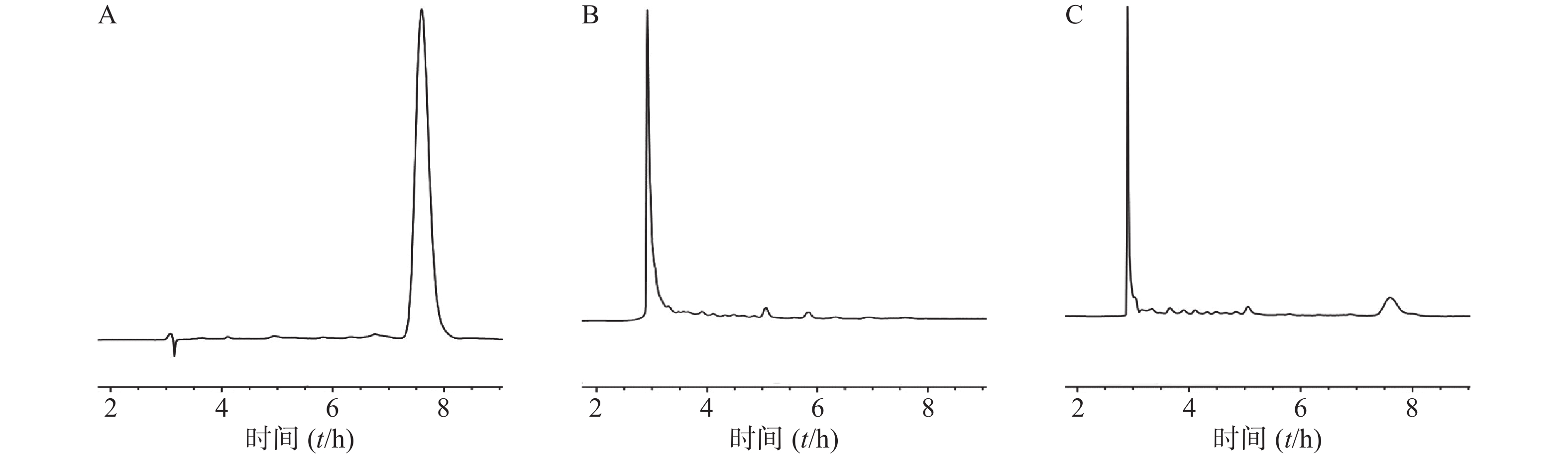
按照“1.6.1”项下色谱条件测定,雷帕霉素出峰良好,保留时间为7.8 min。表明此方法专属性良好(图1)。
-
由表1可知,雷帕霉素在低、中、高3个浓度都具有较好的准确度,日间精密度和日内精密度值均小于5%,表明该方法可用于雷帕霉素的含量测定。
浓度(μg/ml) 日内精密度 日间精密度 检测值(μg/ml) RSD(%) 检测值(μg/ml) RSD(%) 0.50 0.58±0.01 1.62 0.58±0.01 1.13 2.00 1.95±0.02 0.56 1.96±0.01 0.49 10.00 9.36±0.04 0.40 10.02±0.09 0.87 -
由表2可知,雷帕霉素在低、中、高3个浓度的回收率为97.64%~98.62%,符合95%~105%的范围,且RSD值均小于1%,表明该提取方法稳定可靠。
浓度(μg/ml) 检测值(μg/ml) 回收率(%) RSD(%) 0.50 0.57±1.54 113.47 1.69 2.00 1.93±0.02 96.48 0.82 10.00 9.82±0.05 98.21 0.52 -
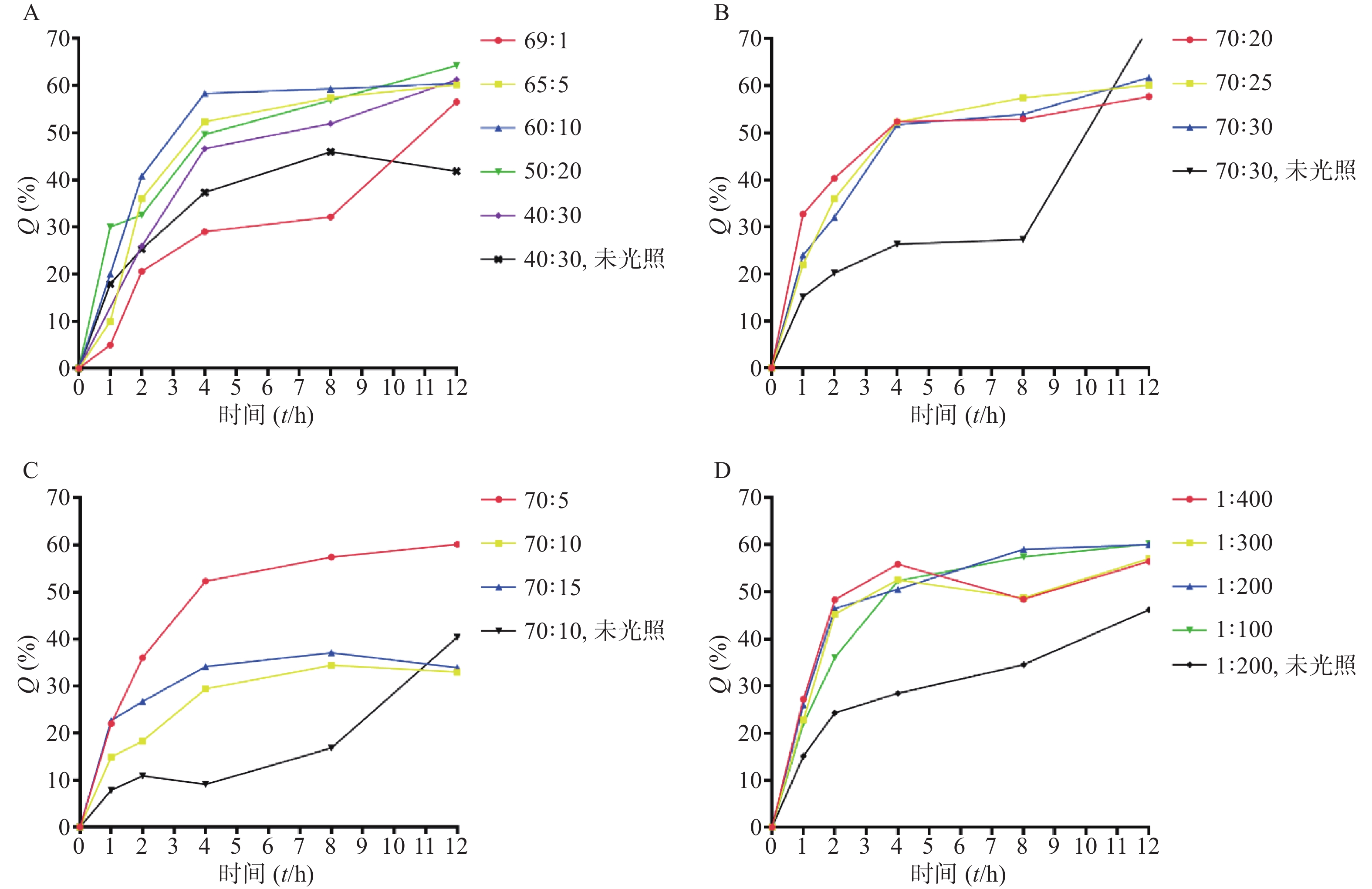
由表3可知,包含不同摩尔量DLPC的处方制得的脂质体,成膜及水化效果较好,粒径<200 nm,PDI<0.2,雷帕霉素包封率>90%,载药量>1%;脂质体体外释放试验,光照12 h后,测得雷帕霉素药物累积释放率>60%(图2A)。当DLPC含量较低时(DSPC∶DLPC=69∶1),雷帕霉素释放速率明显低于其他处方。随着DLPC含量的增加,雷帕霉素释放速率及12 h内累积释放率并无明显增加。
DSPC:DLPC
(mol:mol)成膜 水化 粒径(l/nm) PDI Zeta电位(mV) 包封率(%) 载药量(%) 69∶1 均匀 无沉淀 139.7 0.051 −12.1 98.71 1.11 65∶5 均匀 无沉淀 173.2 0.194 −12.5 94.20 1.23 60∶10 均匀 无沉淀 143.4 0.035 −13.0 98.43 1.15 50∶20 均匀 无沉淀 126.7 0.165 −14.9 92.64 2.27 40∶30 均匀 无沉淀 120.5 0.173 −14.6 92.94 2.22 -
由表4可知,PC(DSPC+DLPC)与胆固醇之比过大(70∶10)或过小(70∶40),脂质体都不能成膜。当PC与胆固醇之比为70∶20、70∶25、70∶30时,所得脂质体成膜及水化效果较好,粒径<200 nm,PDI<0.2,雷帕霉素包封率>90%,载药量在1%左右。在进行脂质体体外释放实验中,测得12 h后雷帕霉素药物累积释放率>60%(图2B)。
PC∶胆固醇
(mol∶mol)成膜 水化 粒径(l/nm) PDI Zeta电位(mV) 包封率(%) 载药量(%) 70∶10 不均匀 — — — — — — 70∶20 均匀 无沉淀 119.4 0.108 −11.0 96.69 0.96 70∶25 均匀 无沉淀 173.2 0.194 −12.5 94.20 1.23 70∶30 均匀 无沉淀 147.7 0.095 −12.7 94.56 0.88 70∶40 不成膜 — — — — — — -
由表5可知,当DSPE-PEG2000用量较小时,脂质体不能成膜。当PC与DSPE-PEG2000之比为70∶5、70∶10、70∶15时,所得脂质体成膜及水化效果较好,粒径<200 nm,PDI<0.2,雷帕霉素包封率>90%,载药量>1%。在进行的12 h脂质体体外释放实验中,摩尔比70∶5组测得雷帕霉素药物累积释放率>60%,其余两组药物累积释放率在33%左右(图2C)。
PC: DSPE-PEG2000
(mol:mol)成膜 水化 粒径(l/nm) PDI Zeta电位(mV) 包封率(%) 载药量(%) 70∶0 不成膜 — — — — — — 70∶1 不成膜 — — — — — — 70∶5 均匀 无沉淀 173.2 0.194 −12.5 94.20 1.23 70∶10 均匀 无沉淀 136.2 0.144 −12.1 98.13 1.37 70∶15 均匀 无沉淀 108.9 0.197 −11.0 97.07 1.34 -
由表6可知,当PdPC(OBu)8用量较大(质量比1∶50),脂质体不能成膜。当PdPC(OBu)8与PC质量之比为1∶100、1∶200、1∶300、1∶400,测得粒径<200 nm,PDI<0.2,雷帕霉素包封率>90%,载药量>1%。在进行的12 h脂质体体外释放实验中,质量比1∶100、1∶200组释放效率高于其余两组,在60%左右(图2D)。
PdPC(OBu)8 :PC
(m:m)成膜 水化 粒径(l/nm) PDI Zeta电位(mV) 包封率(%) 载药量(%) 1∶400 均匀 无沉淀 179.3 0.140 −12.3 93.92 1.15 1∶300 均匀 无沉淀 157.4 0.143 −11.5 94.23 1.16 1∶200 均匀 无沉淀 145.9 0.142 −14.3 93.26 1.19 1∶100 均匀 无沉淀 136.2 0.144 −12.1 98.13 1.23 1∶50 不均匀 沉淀 — — — — — -
DLPC是一种人工合成的不饱和磷脂,在制成的脂质体中,DLPC将均匀分散在脂质双分子层膜上。当有ROS存在时,DLPC结构中的不饱和碳碳双键可被氧化,使脂质膜结构遭到破坏,促进包载药物的释放。光敏剂可在特定波长光照射下产生ROS,在肿瘤疾病的光动力治疗中得到广泛应用。但光敏剂自身潜在的光毒性和溶解性差是其突出缺陷。本文将光敏剂包载在脂质体中可以减少潜在的细胞毒性,同时可增加其溶解度。在研究脂质体中雷帕霉素释放的释放介质确定上,文献[2,4-5]中提供了多种选择。预实验显示,20%乙醇溶液作为释放介质时,不仅克服了脂溶性药物雷帕霉素在水中溶解性差的缺点,同时也使得在进行HPLC检测时干扰峰较少。综上所述,本研究成功制备了光敏ROS响应型雷帕霉素脂质体,表征结果较好。在短时间特定波长照射后,可实现脂溶性药物的快速释放。
Preparation and characterization of photosensitive ROS-responsive rapamycin liposomes
doi: 10.12206/j.issn.2097-2024.202110051
- Received Date: 2021-10-18
- Rev Recd Date: 2022-02-10
- Available Online: 2022-09-29
- Publish Date: 2022-09-25
-
Key words:
- liposome /
- rapamycin /
- near infrared light /
- ROS /
- release in vitro
Abstract:
| Citation: | GAO Xiqing, LU Guangzhao, LU Ying, ZOU Hao. Preparation and characterization of photosensitive ROS-responsive rapamycin liposomes[J]. Journal of Pharmaceutical Practice and Service, 2022, 40(5): 437-441. doi: 10.12206/j.issn.2097-2024.202110051 |








 DownLoad:
DownLoad: