-
转录因子ZNF24(也称KOX17或ZNF191 )是类Krüppel锌指转录因子家族的成员,N端有一SCAN结构域(也称LeR结构域),该区域不仅含有亮氨酸[1],还有选择性的异型或同型寡聚物[2];C端有四个连续的锌指模体且都是典型的类Krüppel样[2]。我们通过小鼠胚胎干细胞基因打靶,获得了ZF-12+/-(又称Zfp191,与ZNF24同源)ES细胞,并将细胞注射入小鼠的囊胚腔,得到了正常发育的ZF-12+/-小鼠,然而得到的ZF-12 −/-小鼠胚胎发育缓慢且在7.5 d左右胚胎致死[2]。最近研究表明,ZNF24通过调控微血管内皮细胞的增殖、迁移和侵袭,在内皮细胞的血管生成中起重要作用[3]。我们前期研究发现ZNF24作为一个因子拥有多种功能,比如参与激酶转录活性调控、血管增殖、大脑发育以及DNA损伤应答等[4]。
ZNF24基因最初由上海交通大学医学院的陈竺院士科研团队与复旦大学的余龙教授科研团队合作从造血细胞中克隆获得,定位于18q12.1[5]。该区域的缺失与人类多种肿瘤相关,如浸润性乳腺癌[6]、结直肠癌[7]等。余龙教授科研团队报道了ZNF24在肝癌中的不同作用:其在肝癌组织中表达上调,可通过与β-连环蛋白基因的启动子结合,激活β-连环蛋白基因转录,进而激活其下游靶基因如细胞周期蛋白 D1 (cyclin D1)基因,促进肝癌细胞的增殖[8];也可直接与DNA甲基转移酶1(DNMT1)启动子结合,激活DNMT1基因转录,引起肝癌细胞DNA甲基化改变,进而激活PI3K-AKT途径促进肝癌细胞增殖[9],提示ZNF24在肝癌中是癌基因。而在转移肝癌组织中ZNF24表达下调,ZNF24通过与DGL1(Discs Large 1) 启动子结合,激活DGL1基因转录,通过Yes相关蛋白(Yes-associated protein, YAP)信号通路抑制肝癌细胞的转移,提示ZNF24在肝癌转移中是抑癌基因[10]。此外,前列腺癌中ZNF24表达上调,通过调控Twist1促进肿瘤细胞上皮间质转换(EMT)、增殖、侵袭和转移,提示ZNF24在前列腺癌中是癌基因[11]。但是,ZNF24在甲状腺癌中表达下调,通过竞争性结合β-连环蛋白,抑制它与辅助因子LEF1/TCF1形成功能性复合物,从而抑制Wnt信号通路,进而抑制肿瘤增生与转移[12],提示ZNF24在甲状腺癌中是抑癌基因。令人感兴趣的是,研究miRNA-940(microRNA-940)在肿瘤中的作用,发现ZNF24是其调控的靶基因,在三阴乳腺癌(TNBC)中miRNA-940靶向下调ZNF24,抑制三阴乳腺癌(TNBC)细胞的增殖和转移[13],提示ZNF24促进TNBC细胞的增殖和转移是癌基因。但是,在人胃癌组织中miRNA-940 通过靶向抑制 ZNF24 表达,促进癌细胞的侵袭和转移[14],提示ZNF24抑制胃癌细胞的侵袭和转移是抑癌基因。这些结果表明,ZNF24通过调控不同的靶基因,在多种不同肿瘤的发生发展、侵袭和转移中起着重要复杂的两面性作用(促进或抑制)。
结直肠癌是一种常见的恶性肿瘤,在我国拥有较高的发病率和较低的生存率[15]。目前,结直肠癌与ZNF24的关系仍不明确。因此,我们构建ZNF24基因过表达的慢病毒载体,包装成病毒并转染结直肠癌细胞HCT116,获得了ZNF24基因过表达的HCT116细胞株,为后续研究的开展提供物质基础。
-
293T细胞、人结直肠癌HCT116细胞、大肠杆菌感受态DH5α均来自本实验室,质粒pMT406、包装质粒pCMV-dR8.9、pCMV-VSV-G均购自上海Sangon Biotech公司。
-
限制性内切酶BamHI(R6021)、限制性内切酶XhoI(RK21100)、DNA胶回收试剂盒(AK1001)、逆转录试剂盒(RR037A)、荧光定量PCR试剂盒(RR420L)(Takara公司,日本);质粒小提试剂盒(PD1211,Promega公司,美国);无缝克隆试剂盒(C5891)、 AxyPrep 总RNA小量提取试剂盒(AP-MN-MS-RNA-250G)、兔抗ZNF24多克隆抗体(D324009)、兔抗GAPDH多克隆抗体(D110016)、山羊抗兔IgG(D111018)(Sangon Biotech公司,中国);BCA蛋白浓度测定试剂盒(P0012A)、胰酶(C0202)(碧云天生物科技公司,中国);DMEM细胞培养基(SH30022,赛默飞世尔生物科技公司,美国);胎牛血清(6170-078, Ausbian公司,澳大利亚)。
-
ZNF24基因、3FLAG和相关引物均由上海Sangon Biotech公司合成。Primer 1和Primer 2用于PCR扩增ZNF24,产物大小为1 128 bp;Primer 3和Primer 4用于PCR扩增3FLAG,产物大小为111 bp; Primer 5和Primer 6用于菌落PCR鉴定,阳性产物大小为1 438 bp。引物序列见表1。
片段名称 序列 Primer 1 TGGCAAAGAATTGGATCCGCC
ACCATGTCTGCACAGTCAGTGGAAGPrimer 2 AACTTTCACAACATTCAGAAGTTTT Primer 3 CTGAATGTTGTGAAAGTTGACTACAAGGATGA Primer 4 CATAATACTAGTCTCGAGTTATTTGTCGTCATCATC Primer 5 CGGCTCTAGAGCCTCTGCTA Primer 6 CGTGAGTCAAACCGCTATCCAC ZNF24(或3FLAG)的PCR反应条件:98 ℃预变性3 min;98 ℃变性10 s,55 ℃退火15 s,72 ℃延伸1 min(或10 s),共 30个循环;72 ℃延伸10 min。
-
用限制性内切酶BamHI和XhoI酶切pMT406,胶回收线性化载体(大小约8 537 bp)。线性化的载体、PCR扩增的ZNF24与3FLAG产物,通过同源重组(无缝克隆)反应,将10 μl反应产物转化至DH5α。平皿培养过夜,挑取单克隆进行菌落PCR鉴定, 阳性克隆产物预期为1 438 bp。PCR阳性的克隆进一步测序鉴定,测序正确的重组质粒命名为pMT-ZNF24。
-
转染前24 h,胰酶消化并重悬293T细胞,取10个10 cm的培养皿,以1×107个/皿的细胞密度铺板。细胞贴壁后将原有培养基更换为Opti-MEM®培养基,体积为9 ml。取100 μg pMT-ZNF24(或pMT406)、65 μg pCMV-dR8.9、35 μg pCMV-VSV-G和适量Opti-MEM®培养基加入至15 ml无菌离心管中混匀,总体积为5 ml。再取500 μl细胞转染液和4.5 ml Opti-MEM®培养基混匀后滴加至上述离心管中,轻柔摇晃至均匀,室温孵育20 min。孵育完成后,将混合液分装到293T细胞中,每皿1 ml,轻轻摇晃混匀后放回培养箱。细胞培养6 h后弃上清液,加入10 ml DMEM培养基继续培养,2 d后收集细胞上清液。用60 ml 0.22 μm PVDF过滤装置过滤上清液, 4 ℃,25 000 r/min离心2 h,然后分装保存于−80 ℃冰箱。采用孔稀释法测定病毒滴度:准备5个EP管,各加入90 μl含10%FBS的高糖DMEM。EP管1中添加10 μl的待测病毒原液,EP管2中添加EP管1混合液10 μl,依次操作至EP管5。293T细胞接种到96孔板的 5个孔中,每孔约5×104个细胞,待细胞贴壁后去掉原液,依次加入EP管中的病毒液继续培养,24 h后换液,观察并记录3 d后稀释率最大孔中的荧光细胞数量。病毒滴度=荧光细胞数/病毒原液量。
-
胰酶消化HCT116细胞,重悬后接种于24孔板中,每孔细胞约为3×105个,待细胞融合达30%时以细胞感染指数(MOI)=10计算病毒浓缩液体积并转染细胞。将HCT116细胞分为3组:空白对照组(HCT116细胞不转染病毒)、阴性对照组(HCT116细胞转染不含ZNF24的空载体慢病毒)和ZNF24组(HCT116细胞转染ZNF24过表达慢病毒)。转染72 h后,在ZNF24组和阴性对照组中加入 4 μg / ml嘌呤霉素,继续培养72 h后得到稳定表达细胞株。
-
慢病毒转染HCT116细胞,TRIzol法裂解细胞并提取RNA,mRNA反转录成cDNA后扩增ZNF24。ZNF24引物序列上游为CATTCCCTAAGGCACTGTGAT,下游为TTGAGGAACACCCATACTGAGA;GAPDH引物序列上游为TGACTTCAACAGCGACACCCA,下游为CACCCTGTTGCTGTAGCCAAA。2−ΔΔCt法分析ZNF24 mRNA的表达量。
-
慢病毒转染HCT116细胞,裂解液(含蛋白酶抑制剂)裂解细胞,提取总蛋白并测定浓度。SDS-PAGE电泳后转模,脱脂牛奶封闭1 h,室温一抗孵育3 h,室温荧光素标记二抗孵育2 h,Odyssey双色红外激光成像系统检测荧光信号。
-
实验数据以3个独立试验的(
$\bar{x} \text{±} s$ )表示,采用 GraphPad Prism 5.0软件中单因素方差分析或t检验进行分析。 -
电泳结果显示,分别获得了大小约1 128 bp的ZNF24扩增产物与大小约111 bp的3FLAG扩增产物(图1)。
-
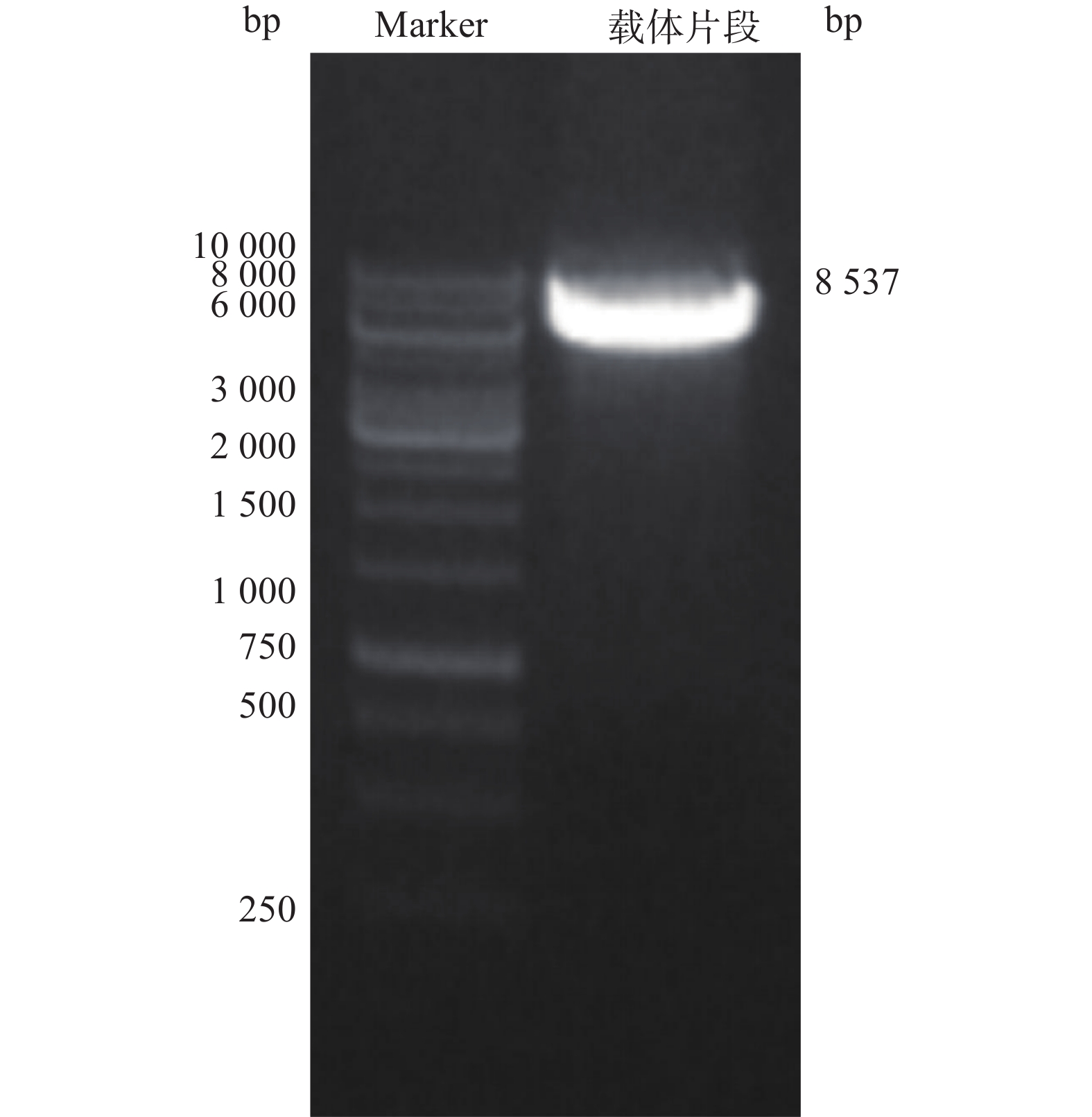
电泳结果显示,得到大小约8 537 bp线性化载体条带(图2)。
-
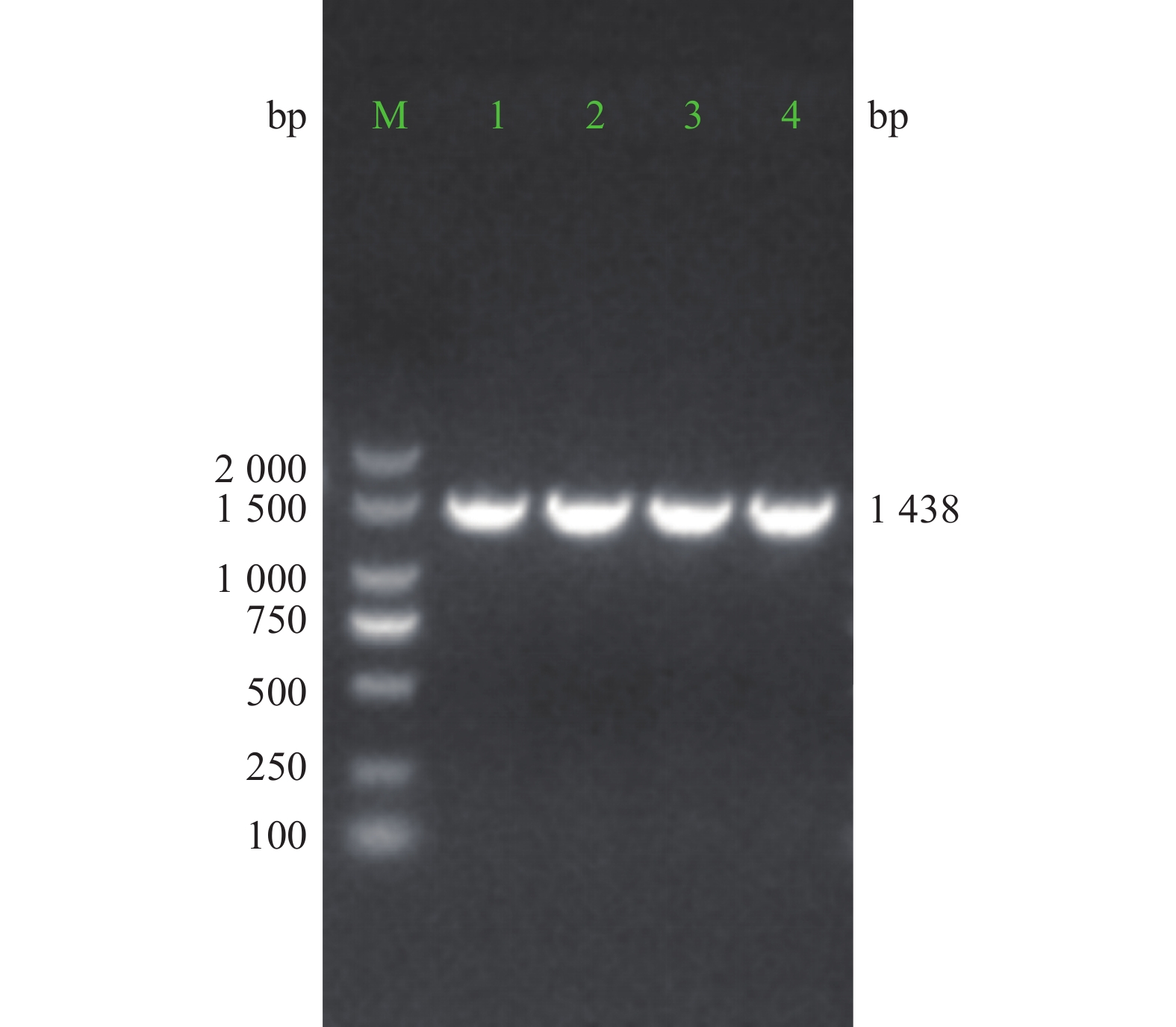
重组质粒经PCR扩增,电泳结果显示,获得约1 438 bp大小的阳性克隆PCR产物条带(图3)。对PCR产物进行测序比对分析,结果与目标序列完全一致。
-
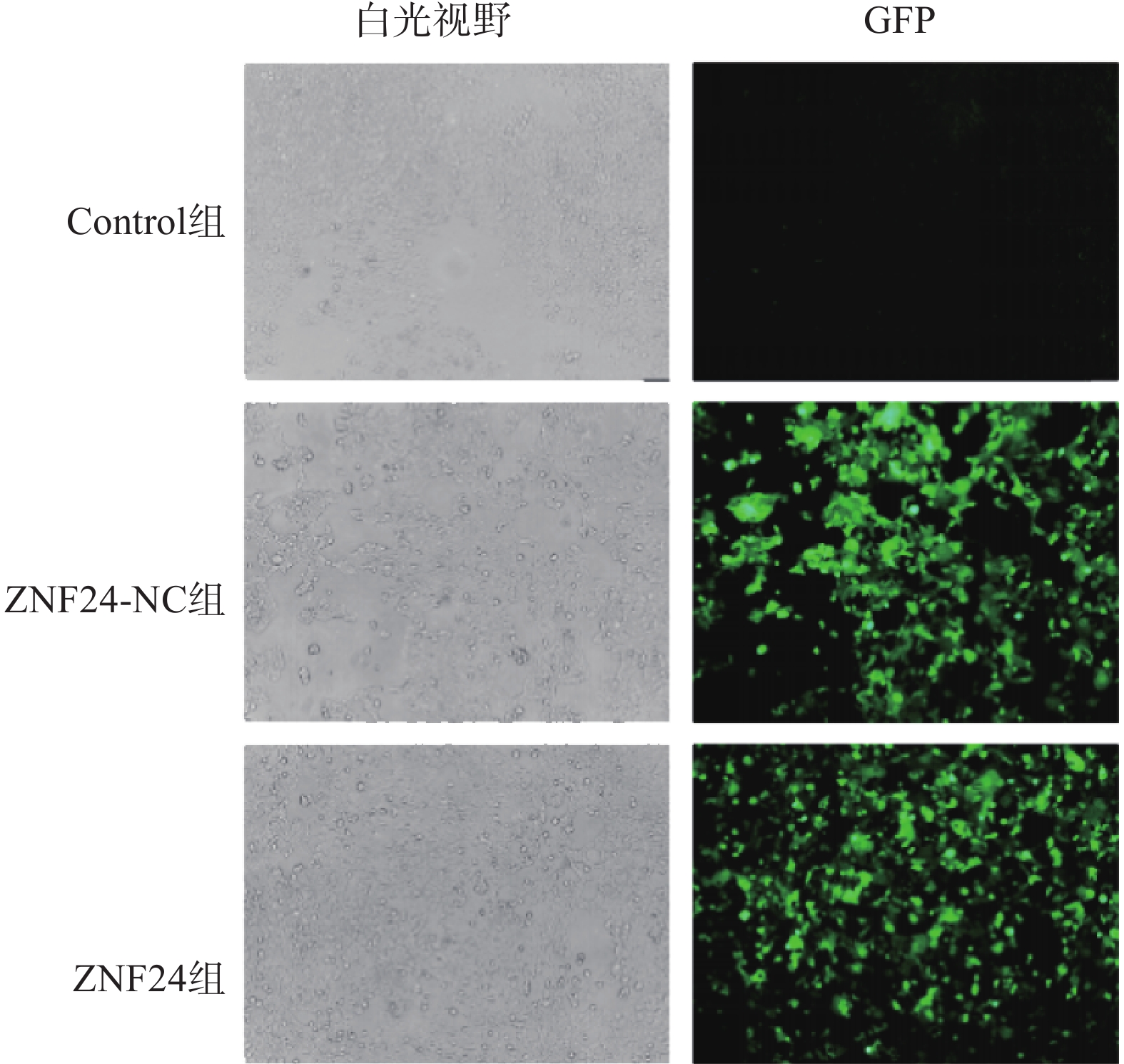
ZNF24过表达慢病毒的滴度为3.25×109 TU/ml,ZNF24-NC慢病毒的滴度为6.19×109 TU/ml。以MOI=10计算病毒体积并转染HCT116细胞,4 μg /ml 嘌吟霉素筛选,荧光显微镜下约85%的细胞呈现绿色荧光蛋白(GFP)表达(图4)。
-
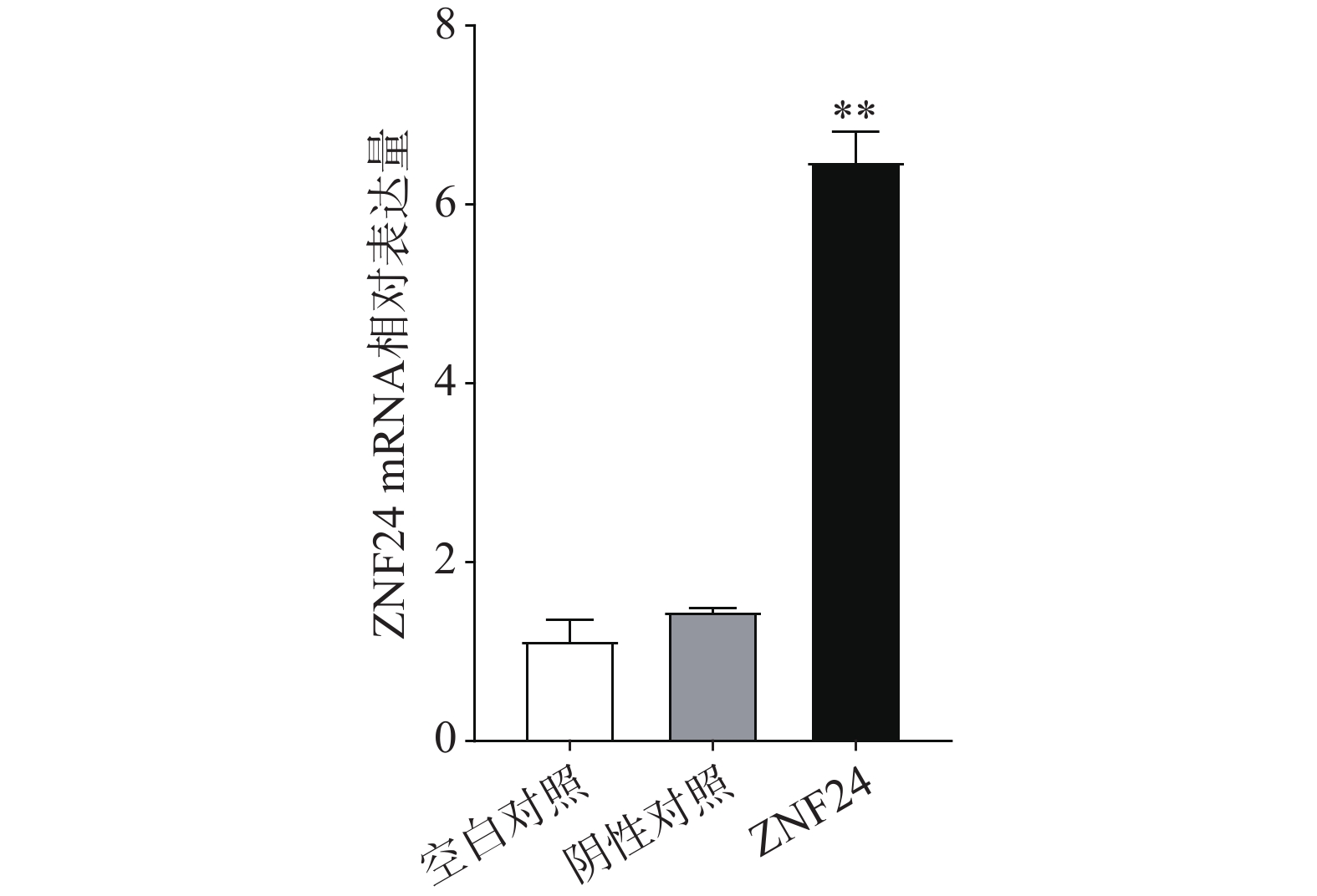
qRT-PCR结果表明,转染ZNF24过表达慢病毒的细胞组中ZNF24的mRNA表达显著高于阴性对照组和空白对照组(图5)。
-
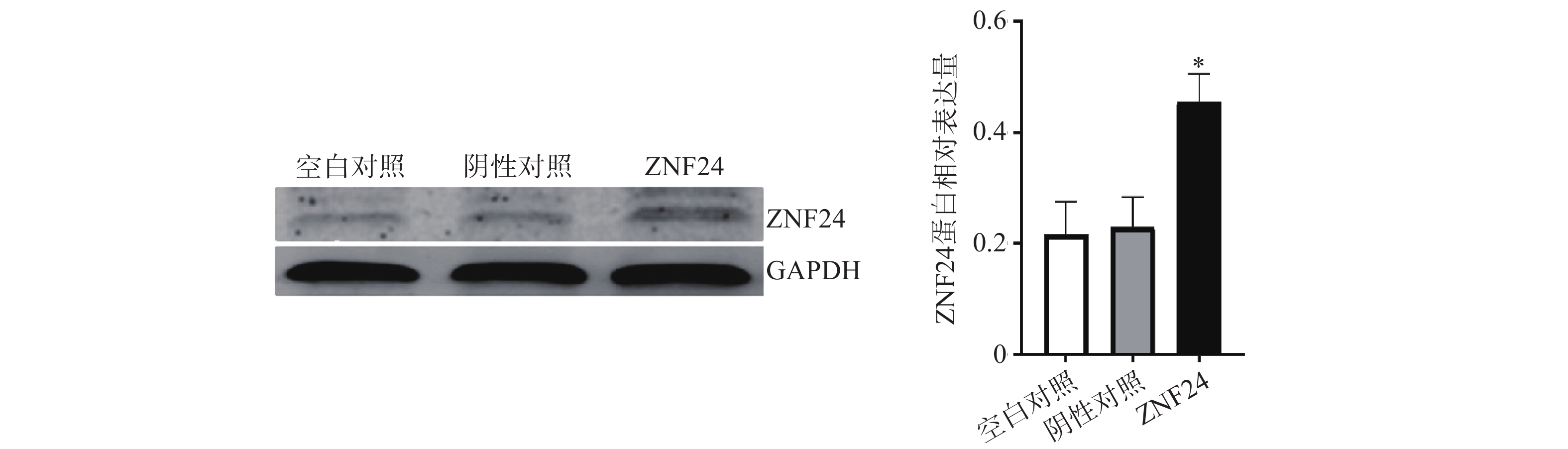
蛋白印迹检测结果显示,转染ZNF24过表达慢病毒的细胞组中ZNF24表达水平明显高于阴性对照组和空白对照组 (图6)。
-
结直肠癌是癌症致死的一个主要原因[16],致死的关键要素是其高水平的复发和转移[17]。结肠癌的发病机制十分复杂,涉及多种癌基因、抑癌基因的异常表达。因此,研究结直肠癌的发病机制,特别是结直肠癌复发转移的机制极其重要。研究表明,多种转录因子在结直肠癌等肿瘤中异常表达,参与结直肠癌的发病、转移和侵袭,如在肿瘤微环境参与VEGF表达调控的 STAT3转录因子已成为新的抗肿瘤药物的作用靶点[18]。
体外与乳腺癌细胞肿瘤动物模型的研究表明,ZNF24通过与血管内皮生长因子(VEGF)启动子上游序列(−144/−134, 非(TCAT) n重复序列)直接结合抑制VEGF基因转录,从而抑制血管增生达到抑制肿瘤生长[19]。然而,敲减人微血管内皮细胞中的ZNF24导致细胞迁移,侵袭和增殖减弱,暗示ZNF24具有促进人微血管内皮细胞的血管生成潜力[3]。多项研究表明ZNF24通过调控不同靶基因(如Twist1[11]、β-连环蛋白[8]和DGL1[10]等)的转录表达以及竞争性结合蛋白因子[8],在多种不同肿瘤的发生发展、侵袭和转移中起着重要复杂的两面性作用(促进或抑制)。
本研究成功构建了ZNF24过表达慢病毒载体,获得相应的病毒。转染HCT116细胞,获得稳定过表达ZNF24的HCT116细胞株,为开展后续研究提供了物质基础。
Construction and expression of lentiviral vector overexpressing ZNF24 gene in human colorectal cancer HCT116 cell
doi: 10.12206/j.issn.2097-2024.202204040
- Received Date: 2022-04-10
- Rev Recd Date: 2022-09-05
- Available Online: 2023-07-14
- Publish Date: 2023-04-25
-
Key words:
- ZNF24 gene /
- colorectal cancer /
- lentiviral vector
Abstract:
| Citation: | TIAN Shuo, LI Jianzhong. Construction and expression of lentiviral vector overexpressing ZNF24 gene in human colorectal cancer HCT116 cell[J]. Journal of Pharmaceutical Practice and Service, 2023, 41(4): 222-226. doi: 10.12206/j.issn.2097-2024.202204040 |










 DownLoad:
DownLoad: