-
高原是指海拔高于2 500 m能够引起机体产生明显生物学效应的地区[1]。高原低氧环境会影响机体的生理功能与药物疗效的发挥[2]。高原地区高血压患病率显著高于平原地区,高血压是高原地区最常见的慢性疾病和心血管疾病的严重危险因素。临床上最常使用钙通道阻滞剂类降压药(CCB)[3],硝苯地平是治疗高血压的一线用药,通过阻断血管平滑肌细胞上的钙通道从而扩张血管降低血压,同时阻断心肌细胞L型钙通道从而减慢心率[4]。本课题组前期研究表明,急进高原后大鼠体内硝苯地平的药动学参数会发生显著变化[5]。由于高原低氧环境导致药物的药动学参数改变,体内血药浓度可能达不到有效治疗浓度,从而影响硝苯地平降压效果,但目前有关硝苯地平对高原高血压人群的疗效研究尚未见报道。本研究首次报道高原低氧环境下硝苯地平控释片对高血压患者降压作用的临床疗效及安全性评价,从而为高原高血压人群提供有效治疗方案,为临床合理用药提供参考。
-
研究对象为2020年2月至2021年2月于中国人民解放军联勤保障部队第九四〇医院及玉树八一医院诊治的共84例高血压患者。本研究经第九四〇医院伦理委员会批准,审批编号为2019KYLL068。
-
①符合《中国高血压防治指南》中关于高血压的诊断标准[6],即非同日3次所测量的诊室血压:收缩压(SBP)≥140 mmHg和(或)舒张压(DBP)≥90 mmHg的患者;②高原高血压患者为久居高原1年及以上者,平原高血压患者为久居平原1年及以上者,两组患者近1个月内未服用降压药物;③入院后连续每日晨起服用硝苯地平控释片30 mg,连续6 d达到稳态血药浓度者;④年龄40~85岁。
-
①继发性高血压或临床诊断为病危者;②伴发有严重心脑血管疾病者。③肝功能异常者,丙氨酸氨基转移酶(ALT)或天冬氨酸氨基转移酶(AST)超过上限1.5倍者;④肾功能异常者或存在影响肾功能的急性因素者。
-
硝苯地平控释片,规格:30 mg/片,批号:IX20200382,批准文号:国药准字J20180025,德国Bayer AG公司生产。
-
将42例第九四〇医院高血压住院患者(海拔1 500 m)设为平原高血压组,42例八一医院高血压住院患者(海拔3 800 m)设为高原高血压组。两组患者均给予硝苯地平控释片每日晨起口服30 mg,连续6 d。
-
根据我国总结和规定的降压药临床研究指导原则:将血压作为药效评价的主要指标,将记录的平原与高原组血压值,按照疗效标准等级:显效、有效、无效进行评价。考察相同剂量下,高原低氧环境对硝苯地平降压疗效的影响。监测平原高血压组和高原高血压组患者按照每日晨起口服30 mg硝苯地平控释片连续6 d的血压值,包括每天清晨、服药前及临睡前3个时段的SBP和DBP。具体操作为:在患者平躺情况下,对每个时间段的每次血压均测定3次,计算出平均值。疗效判定按文献[7-8]的方法对3个时间段的血压进行评价,分为显效、有效和无效。显效:SBP下降幅度≥10 mmHg并降至正常或SBP未达正常但下降幅度≥20 mmHg;有效:SBP下降1~10 mmHg并降至正常或SBP未降至正常但下降10~19 mmHg;无效:未达到上述标准。对总有效率(总有效率=显效率+有效率)进行统计学比较,以判定疗效。
此外,临床研究显示,较快的心率会显著增加高血压患者心血管事件和病死率[9-11]。依据专家建议,高血压患者应首先控制血压达标,在降压治疗的同时注重心率管理[12]。硝苯地平控释片具有反射性刺激交感神经激活的作用,可能导致心率加快引起心悸等不良反应。根据国内外指南和专家共识,在测定血压时一并测得心率,详细记录清晨、服药前、临睡前的心率。
-
用SPSS Statistics 21进行统计分析。计量资料用
$ \bar x \pm s $ 表示,组间比较用独立样本t检验,组内比较用卡方检验;计数资料用率表示,比较用卡方检验。 -
调查内容包括一般人口学资料、身高、体重、BMI、ALT、AST、Cr等。平原高血压组及高原高血压组的AST、ALT、Cr值均符合纳入排除标准,患者伴发疾病及合并用药较少。两组患者的一般资料比较,差异均无统计学意义(P>0.05),组间具有可比性,见表1。
项目 平原高血压组 高原高血压组 性别(男/女) 30/12 22/20 年龄 61.24±8.02 60.19±9.28 身高(l/cm) 168.79±9.09 168.31±6.45 体重(m/kg) 69.30±10.64 67.29±7.36 BMI (kg/m2) 24.23±2.45 23.80±2.54 AST(U/L) 21.31±8.87 25.14±9.77 ALT(U/L) 27.14±16.90 28.38±15.37 Cr(μmol/L) 74.93±17.33 76.17±19.79 -
依据临床血压监测指导原则,测定了3个不同时间段的血压值,分别为清晨、用药前及临睡前的血压。治疗前,高原高血压组的清晨DBP、服药前及临睡前的SBP和DBP均显著高于平原高血压组,且高原组舒张压升高更为显著。连续用药6 d后,平原高血压组的清晨、服药前及临睡前SBP均显著低于治疗前(P<0.01)。高原高血压组治疗前后的各时间段SBP和DBP均显著高于平原高血压组,差异均有统计学意义(P<0.05),但高原组治疗后与治疗前相比无显著性差异,且血压未降至正常,可见高原高血压组服用硝苯地平在6 d内的降血压疗效比平原高血压组差,详见表2。
组别 检测时间 SBP DBP 清晨 服药前 临睡前 清晨 服药前 临睡前 平原高血压组 治疗前 151.14±15.70 146.90±10.45 148.93±12.39 89.43±13.11 87.48±10.30 89.62±11.60 治疗后 138.86±14.66## 137.93±14.44# 137.95±17.82# 86.90±12.52 86.05±14.06 85.05±12.70 高原高血压组 治疗前 154.55±12.29 153.38±11.66** 154.60±11.98* 104.67±17.18*** 106.86±15.09*** 106.64±14.18*** 治疗后 148.93±19.76* 148.55±19.28** 149.10±20.00** 100.07±16.11*** 102.19±16.60*** 101.86±15.89*** *P<0.05, **P<0.01, ***P<0.001,与平原高血压组比较;#P<0.05,##P<0.01,与治疗前比较。 采用配对样本t检验判断硝苯地平治疗前后对平原高血压组和高原高血压组患者血压的影响,表3结果显示,平原高血压组治疗前后的清晨、服药前及临睡前SBP、临睡前DBP有统计学差异,高原高血压组治疗前后各时间段的SBP和DBP无统计学差异。
组别 SBP下降值 DBP下降值 清晨 服药前 临睡前 清晨 服药前 临睡前 平原高血压组 12.29±17.10*** 8.98±15.40** 10.98±17.70*** 2.52±10.56 1.43±12.36 4.57±10.43** 高原高血压组 5.62±16.72 4.83±19.17 5.50±19.58 4.59±16.94 4.67±17.91 4.78±17.59 **P<0.01, ***P<0.001,与治疗前清晨、服药前、临睡前比较。 -
专家建议将研究结束时给药间隔末(谷值时)血压与基线血压的差值作为主要疗效指标[8],本研究中选用服药前时间段的治疗前后血压之差进行疗效评价。高原高血压组和平原高血压组的总有效率分别为47.62%(20例/42例)和76.19%(32例/42例),高原高血压组硝苯地平控释片降压总有效率显著低于平原高血压组(P<0.05),见表4。
组别 显效 有效 无效 总有效率 平原高血压组 24(57.14) 8(19.05) 10(23.81) 32(76.19) 高原高血压组 13(30.95) 7(16.67) 22(52.38) 20(47.62)* *P<0.05,与平原高血压组比较。 两组患者均按照医嘱连续服药,获得81例患者不同时段心率数据。其中,平原高血压组41例,高原高血压组40例。高原高血压组各时间段的心率均显著高于平原高血压组,证实高原低氧环境会导致心率显著上升。连续用药6 d后,平原高血压组的清晨及服药前心率显著低于治疗前(P<0.05),高原高血压组心率有所下降,但无显著性差异(P>0.05),见表5。提示应在降压治疗的同时关注心率管理,以预防心血管事件的发生。
组别 治疗前 治疗后 清晨 服药前 临睡前 清晨 服药前 临睡前 平原高血压组 109.51±17.46 110.37±17.90 109.85±17.62 100.46±21.13# 101.46±21.88# 101.39±21.42 高原高血压组 119.03±13.96** 119.70±14.64* 119.43±14.05** 113.65±20.22** 113.18±19.56* 114.55±19.55** *P<0.05,**P<0.01,与平原高血压组比较;#P<0.05,与治疗前比较。 -
在试验过程中,平原高血压组5例患者发生心动过速、心悸的症状,高原高血压组6例患者发生心动过速、心悸的症状。两组患者的药物不良反应发生率分别为11.90%和14.29%,差异无统计学意义(P>0.05)。
-
本研究结果表明,高原高血压组的血压和心率值均显著高于平原高血压组,且高原高血压组血压控制不良、心率管理不佳,高原高血压患者服用与平原高血压患者同剂量的硝苯地平控释片未能有效地控制短期内血压。高原地区硝苯地平用药剂量应高于平原剂量,因此有必要建议《中国高血压防治指南》可增加一项高原地区高血压人群用药剂量。以增加药物在机体内的血药浓度,从而长期有效控制血压,与此同时需做好高血压患者服药前后血压的监测及患者安全用药教育,减少药物不良反应的发生,从而提高高原地区用药的合理性、安全性及有效性。
高原合理用药展望,目前,高原地区的合理用药指导有待提高,原因有以下几点:①地域偏远、文化差异及经济欠发达导致高原地区医护人员的合理用药意识存在局限性。此外,高原地区高血压的患病率高于全国平均水平,但患者的知晓率、治疗率和控制率明显偏低[13]。②高血压是心血管系统中最常见的慢性疾病,可诱发许多并发症,高血压严重危害高原地区人群的生命健康,降低血压可随之降低心血管疾病并发率,改善预后[14-15],但高原地区患者服药的依从性差,不能长期遵医嘱服药,导致血压控制不佳,增加心血管疾病患病风险。③高原低氧环境会改变药物药动学参数从而影响药物疗效,但高原地区人体的药动学研究少有报道。从事高原合理用药研究的相关人员较少,高原人群的特殊用药至今未列入研究的议事日程。高原低氧环境导致药动学改变从而导致药效的差异是个体化合理用药中必须考虑的问题。综上,针对高原地区沿用平原临床用药方案的问题亟需解决,应着重研究高原与平原地区合理用药的临床疗效差异,发布高原地区合理用药指南,培养和提高高原地区医护人员合理用药意识,提高患者对疾病的知晓率、治疗率和控制率,提高患者用药依从性。
Clinical trial of nifedipine controlled-release tablets on reducing blood pressure in the treatment of patients with hypertension at high altitude
doi: 10.12206/j.issn.2097-2024.202205112
- Received Date: 2022-05-30
- Rev Recd Date: 2022-09-01
- Available Online: 2022-09-29
- Publish Date: 2022-09-25
-
Key words:
- nifedipine controlled-release tablet /
- high altitude hypoxia /
- hypertension /
- clinical efficacy
Abstract:
| Citation: | HUANG Qin, GAO Zizhao, NIYANG Zhuoma, SUONAN Gele, WANG Rong. Clinical trial of nifedipine controlled-release tablets on reducing blood pressure in the treatment of patients with hypertension at high altitude[J]. Journal of Pharmaceutical Practice and Service, 2022, 40(5): 395-398. doi: 10.12206/j.issn.2097-2024.202205112 |








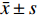
 DownLoad:
DownLoad: