-
苯磺酸顺阿曲库铵为临床常用的中长效肌肉松弛药,苏醒期药物在体内的残留导致残余肌松的发生,成为导致麻醉后发生呼吸系统不良事件的主要原因,尤其是老年患者,术后残留会带来更严重的不良后果[1],因此对老年患者手术后血浆中苯磺酸顺阿曲库铵的残留进行监测有重要意义。苯磺酸顺阿曲库铵易发生霍夫曼降解,温度和pH值的升高会加速降解[2],因此,临床收集的血浆样本的稳定保存至关重要。本研究在既往文献报道的HPLC法及高效液相色谱-质谱(LC-MS)法[3-6]基础上,开发了一种用灵敏、准确、高效的超高效液相色谱-串联质谱(UPLC-MS/MS)测定人血浆中苯磺酸顺阿曲库铵浓度的方法,有效避免了苯磺酸顺阿曲库铵的降解,对老年患者手术后血浆中苯磺酸顺阿曲库铵浓度随时间变化的规律进行研究,探讨自体血回输对患者体内苯磺酸顺阿曲库铵浓度的影响。
-
Agilent 1290 UPLC超高效液相色谱(安捷伦,美国);Agilent 6470三重四极杆质谱,配备AJS-ESI喷射流电喷雾离子源(安捷伦,美国);SECURA125-1CN型电子天平(赛多利斯,德国);Arium® mini 超纯水机(赛多利斯,德国)。
-
苯磺酸顺阿曲库铵对照品(中国食品药品检定研究院,批号101170-201902,纯度>98%);内标维库溴铵对照品(中国食品药品检定研究院,批号100813-201603,纯度>99%);甲酸、乙酸铵为色谱纯(麦克林,中国);乙腈为质谱纯(霍尼韦尔,美国);水为超纯水。
-
色谱柱为SHISEIDO(资生堂)ADME(3.0 mm×100 mm,2.7 μm),柱温35 ℃;流动相系统以含0.1%甲酸的2 mmol/L乙酸铵溶液为A相,以乙腈溶液为B相,等度洗脱,A∶B=30∶70(V/V),流速:0.35 ml/min;进样量2 μl,分析时间3 min。
-
选择AJS-ESI离子源正离子模式,离子源参数设置:雾化器压力40 psi;干燥气流速11 L/min;干燥气温度350 ℃;鞘气流速11 L/min;鞘气温度350 ℃;毛细管电压4 000 V。
多重反应监测(MRM)参数:苯磺酸顺阿曲库铵464.3→358.2(m/z),碎片电压135 V,碰撞能量20 eV,保留时间为2.05 min;维库溴铵(IS)557.4→356.3(m/z),碎片电压135 V,碰撞能量35 eV,保留时间为1.86 min。
-
取苯磺酸顺阿曲库铵对照品约1.5 mg,精密称定,置于2 ml棕色称量瓶中,精密加入含0.1%甲酸的乙腈溶液溶解并涡旋混匀,配成浓度为1.0 mg/ml的储备液;取上述储备液适量,用含0.1%甲酸的乙腈溶液按比例逐级稀释,配成浓度为400~100 000 ng/ml的标准溶液,置于4 ℃冰箱备用。
同法配制浓度为800、40 000、80 000 ng/ml的质控溶液,置于4 ℃冰箱备用。
-
取维库溴铵对照品约1.5 mg,精密称定,置于2 ml棕色称量瓶中,精密加入含0.1%甲酸的乙腈溶液溶解并涡旋混匀,配成浓度为1.0 mg/ml的内标储备液;取上述储备液用含0.1%甲酸的乙腈溶液稀释为500 ng/ml的内标标准溶液,置于4 ℃冰箱备用。
-
精密吸取空白人血浆与不同浓度的苯磺酸顺阿曲库铵标准溶液,配制为浓度为20、50、100、250、500、1 000、2 500、5 000 ng/ml的苯磺酸顺阿曲库铵模拟标准曲线样品。同法配制浓度为40、2 000、4 000 ng/ml的质控样品。
-
取血浆样品50 µl,加入50 µl的维库溴铵内标标准溶液、400 µl的含0.1%甲酸的乙腈溶液,涡旋1 min, 13 000 r/min(5 ℃)离心5 min,取上清液100 µl进样。
-
该临床试验经上海市浦东新区公利医院医学伦理委员会审批同意,批件号:(2020)临审第(018)号。选取老年脊柱手术患者给予咪达唑仑0.05 mg/kg,丙泊酚1.5 mg/kg、舒芬太尼0.5 μg/kg、苯磺酸顺阿曲库铵0.2 mg/kg麻醉诱导;诱导后进行急性等容性血液稀释(ANH),采集总血容量的10% 血液冷藏保存于ACD储血袋中;术中丙泊酚2.5 μg/ml、瑞芬太尼0.1~0.2 μg/(kg·min)、苯磺酸顺阿曲库铵2.0 μg/(kg·min)维持麻醉,于手术主要步骤完成后停止泵注苯磺酸顺阿曲库铵并回输采集的自体血液。收集麻醉诱导结束(T1)、术毕(T2)、术毕0.5 h(T3)、术毕1 h(T4)、回输时刻ACD血袋(LT)的血样各5 ml,于3 000 r/min离心5 min,分离得血浆,精密吸取1 ml血浆置于事先加入100 µl的2%甲酸水溶液的冻存管中,振摇10 s混合后,于−80 ℃冰箱保存。
-
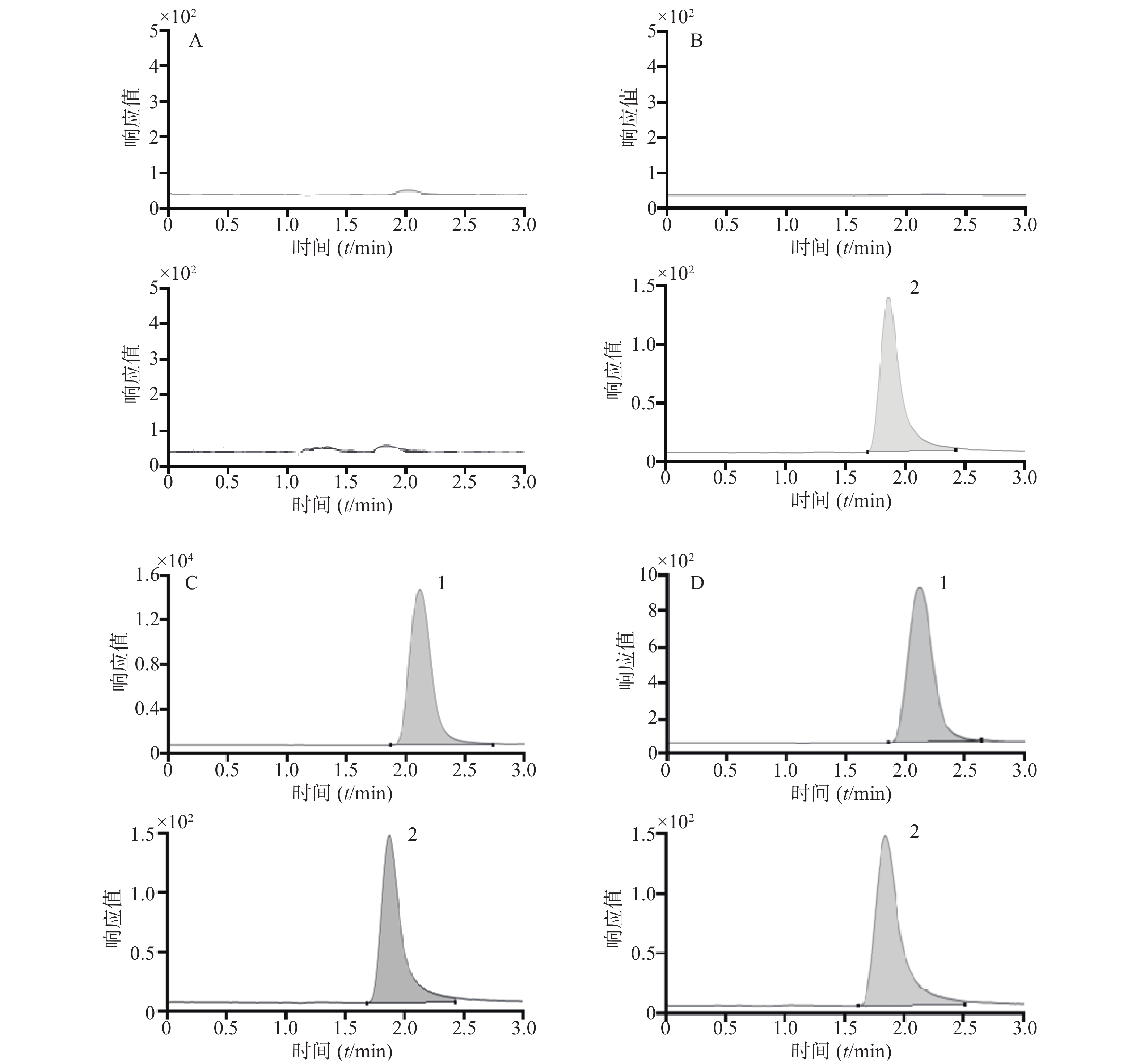
取空白人血浆样品、模拟血浆样品(苯磺酸顺阿曲库铵浓度为5 000 ng/ml)以及手术中采集的人血浆样品,分别按“2.1.4”项下进行血浆样品前处理后进样,获得相应的零点样品、模拟样品及人血浆样品的色谱图;空白人血浆样品处理时以含0.1%甲酸的乙腈溶液代替内标溶液,获得双空白样品的色谱图。结果表明该方法选择性良好,见图1。
-
以血浆中苯磺酸顺阿曲库铵浓度为X,苯磺酸顺阿曲库铵与维库溴铵的峰面积比值为Y,用1/X权重求得回归方程:Y=0.002 6 X+0.010 9,(r=0.999 7),表明在20~5 000 ng/ml范围内线性关系良好。
-
通过在定量上限样品(浓度为5 000 ng/ml)之后立即进样分析双空白样品来评估系统残留。结果显示双空白样品中苯磺酸顺阿曲库铵的峰面积不超过该分析批定量下限样品待测物峰面积的20.0%,内标维库溴铵的峰面积不超过该分析批标准曲线内标最小峰面积的5.0%,表明该方法的残留效应不影响测定。
-
进样分析低、中、高3个浓度(40、2 000、4 000 ng/ml)的质控样品,每个浓度平行操作6份,连续分析3 d,计算批内、批间的精密度(RSD)及准确度(RE),结果表明批内和批间的RSD<10%,RE<±10%,见表1。
浓度(ng/ml) 批内(n=6) 批间(n=18) 精密度
(RSD,%)准确度
(RE,%)精密度
(RSD,%)准确度
(RE,%)40 3.10 1.34 7.66 −7.67 2 000 8.27 −5.98 5.23 −7.75 4 000 6.47 4.62 9.34 −5.17 -
配制两种溶液,其中苯磺酸顺阿曲库铵和维库溴铵的浓度均与低、中、高浓度质控样品处理后的浓度一致:①将6个不同来源的空白人血浆按“2.3.1”项下处理为双空白样品后,加入苯磺酸顺阿曲库铵和维库溴铵对照品溶液;②配制两者的对照品溶液。分别记录两种溶液分析后苯磺酸顺阿曲库铵和维库溴铵的峰面积,以峰面积的比值计算内标归一化的基质效应。
再配制2种溶液:①将低、中、高3个浓度水平的质控样品按“2.1.4”项下处理;②将单一来源的空白人血浆按“2.3.1”项下处理为双空白样品后,加入苯磺酸顺阿曲库铵和维库溴铵对照品溶液,使两者浓度均与低、中、高浓度质控样品处理后的浓度一致。分别记录两种溶液分析后苯磺酸顺阿曲库铵和维库溴铵的的峰面积,以峰面积的比值计算提取回收率。
结果表明,低、中、高浓度的基质效应影响一致,RSD为5.81%,基质效应和提取回收率的考察结果见表2。
品名 浓度
(ng/ml)内标归一化的
基质效应(%)提取回收率
(%)苯磺酸顺阿曲库铵 40 71.88 83.62 2 000 77.67 83.40 4 000 80.64 88.87 维库溴铵 125.91 -
将新鲜配制的苯磺酸顺阿曲库铵和维库溴铵的储备液(浓度均为1.0 mg/ml)于4 ℃冰箱中放置28 d,以峰面积的偏差考察其稳定性,结果显示苯磺酸顺阿曲库铵峰面积的偏差为−4.37%,维库溴铵峰面积的偏差为4.75%,表明待测物和内标的储备液稳定性良好。再考察低、中、高3个浓度水平的质控样品(浓度为40、2 000、4 000 ng/ml),室温下放置3 h后处理、处理后在自动进样器(4 ℃)放置24 h、反复冻融3次、−80 ℃冰箱中放置30 d的稳定性,每个浓度平行操作6份,结果见表3。
浓度
(ng/ml)室温(n=6) 进样器(n=6) 冻融(n=18) 长期(n=6) 精密度(RSD,%) 准确度(RE,%) 精密度(RSD,%) 准确度(RE,%) 精密度(RSD,%) 准确度(RE,%) 精密度(RSD,%) 准确度(RE,%) 40 6.23 7.30 6.75 −1.86 3.89 −2.19 2.23 8.24 2 000 1.38 0.33 2.57 −7.87 4.72 −8.99 3.62 −12.62 4 000 6.75 −2.25 2.40 −6.04 4.38 −10.96 3.48 −10.89 -
研究纳入8名老年患者,受试者1~4诱导后进行ANH,受试者5~8未进行ANH。采用建立的方法测定临床采集的血浆样本的浓度,结果见表4。
时间点(t/h) 受试者1 受试者2 受试者3 受试者4 受试者5 受试者6 受试者7 受试者8 T1 332.0 362.3 556.8 388.9 289.5 328.1 359.9 348.3 T2 228.4 190.9 363.6 205.9 65.71 180.3 170.1 158.5 T3 81.66 99.30 174.4 145.9 41.60 68.56 73.07 61.64 T4 50.11 58.34 125.9 84.42 24.72 35.21 40.14 34.22 LT 270.8 338.0 489.9 316.3 -
既往文献报道的苯磺酸顺阿曲库铵血药浓度的检测方法主要有HPLC法及LC-MS法,但往往需要经过真空离心浓缩或氮吹浓缩等较为复杂的样本前处理方法或经梯度洗脱而分析时间较长[3-6]。本研究在此基础上,开发了UPLC-MS/MS检测方法,优化了前处理方法,采用蛋白沉淀法,操作简便,基质效应和提取回收率满足方法学要求;同时筛选了不同的流动相,最终确定含0.1%甲酸的2 mmol/L乙酸铵溶液∶乙腈(30∶70,V/V)等度洗脱,使苯磺酸顺阿曲库铵的离子化程度达到最优,方法验证结果显示该方法的精密度和准确度符合生物样本的分析要求。
苯磺酸顺阿曲库铵易发生霍夫曼降解而消除,温度和pH值的升高会加速其降解[2],如不加入酸调节pH值,即使在−80 ℃的储存条件下,其仍会很快降解。本研究中,经过对不同浓度甲酸溶液的考察,最终确定标准品的配制溶剂与蛋白沉淀剂均使用含0.1%甲酸的乙腈溶液、血浆样品的收集过程中加入含2%甲酸的水溶液,稳定性研究显示储备液可在4 ℃稳定储存28 d(RE=−4.37%),血浆样本可在−80 ℃稳定储存30 d(低浓度RE=8.24%,中浓度RE=−12.62%,高浓度RE=−10.89%),有效避免了苯磺酸顺阿曲库铵的降解。
残余肌松导致患者出现主观不适,导致低氧或高二氧化碳血症、咽部肌肉无力,严重的出现上呼吸道梗阻影响患者生命安全[7]。国内一项多中心的纳入1 571例腹部手术患者的研究提示,术后肌松残余率高达52.8%[8],而国外一项使用新斯的明拮抗残余肌松的研究显示,拮抗后仍有63.5%的患者在拔除气管导管时存在残余肌松[9]。ANH常规在麻醉诱导后采集稀释血液,所以其中含有一定量的麻醉药物,本研究中亦检测到离体自体血在回输时刻血袋内含有较高浓度的苯磺酸顺阿曲库铵。回输采集自体血后,患者体内苯磺酸顺阿曲库铵浓度较未进行ANH自体血回输的患者浓度下降缓慢,存在残余肌松的风险。尹磊等[10]研究亦显示,针对老年患者在术毕回输ANH采集自体血存在肌松恢复延迟的现象。因此,针对ANH回输自体血的患者,有必要监测苯磺酸顺阿曲库铵的血浆药物浓度,避免围术期不良事件发生。而本研究建立的检测方法有效、稳定,可以用于临床监测苯磺酸顺阿曲库铵的浓度。
Monitoring of residual cisatracurium besylate in elderly patients plasma at post operation by UPLC-MS/MS
doi: 10.12206/j.issn.2097-2024.202206023
- Received Date: 2022-06-07
- Rev Recd Date: 2022-10-12
- Publish Date: 2023-03-25
-
Key words:
- UPLC-MS/MS /
- cisatracurium besylate /
- content determination /
- residual curarization
Abstract:
| Citation: | XU Jiaming, ZHOU Xiaofang, GUO Jianrong. Monitoring of residual cisatracurium besylate in elderly patients plasma at post operation by UPLC-MS/MS[J]. Journal of Pharmaceutical Practice and Service, 2023, 41(3): 192-196. doi: 10.12206/j.issn.2097-2024.202206023 |








 DownLoad:
DownLoad: