-
内生真菌经过长期进化与宿主植物形成互利共生的关系,植物可以为内生真菌提供生长所需的营养物质,内生真菌可以促进植物生长、协助对抗干旱与虫害等胁迫、产生结构新颖丰富的次生代谢物以及诱导植物次生代谢物积累[1]。植物内生真菌作为来源于药用植物的一种新兴微生物资源,在医药、农业和食品工业领域都展现出越来越广泛的应用前景[2]。
金线莲Anectochilus roxburhii为兰科金线兰属(Anectochilus)多年生草本植物,又名金线兰、金耳环、鸟人参等,在民间有“药王”、“金草”等美称,具有清热凉血、除湿解毒等功效,主产于我国南部如福建、台湾、浙江等地,富含多糖、内酯苷和黄酮及苷类等成分,具有抗炎、提高免疫力、保肝、抗肿降糖等生物活性[3, 4]。目前,有关金线莲内生菌的研究多聚焦于植株体内内生菌生物多样性[5]、促进宿主生长[6],以及提高宿主抗病性[7]等研究。对于金线莲内生菌次生代谢产物的研究相对有限[8, 9],从金线莲内生真菌次生代谢产物中发现结构新颖、具有显著药效活性的先导化合物具有较大潜能,有待进一步挖掘。
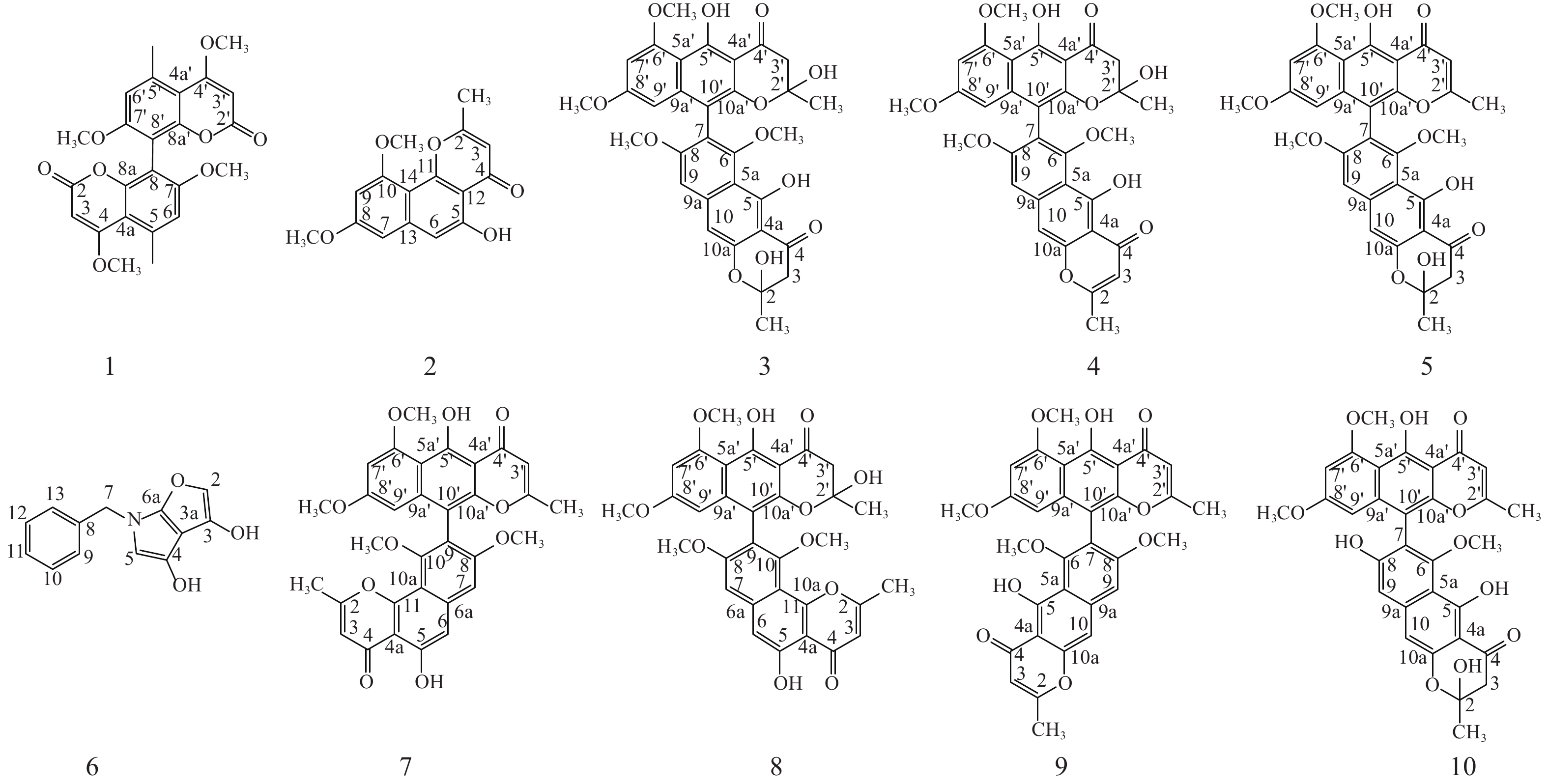
本研究选取从金线莲中分离得到的一株内生真菌株Aspergillus sp. J218,采用大米固体发酵法进行发酵,并从发酵产物的乙酸乙酯萃取物中分离纯化、鉴定得到10个化合物(图1)。所鉴定成分多为二聚萘并吡喃酮类化合物,提示该株内生真菌含有丰富的二聚化合成酶,对其生物合成潜能有进一步挖掘的价值。
-
Agilent 1200 Series 半制备型液相色谱仪(美国Agilent公司);Eclipse XDB-C18 半制备色谱柱(250 mm×9.4 mm,5 μm;美国Agilent公司);Bruker Avance 600 MHz(德国Bruker公司);APEX7.0 FT-MS型质谱仪(德国Bruker公司);RE-52C旋转蒸发仪(上海沪西分析仪器厂有限公司);HWS-24型恒温水浴锅(上海一恒科学仪器有限公司);SHZ-DⅢ型循环水式真空泵(巩义予华仪器有限责任公司);Sephadex LH-20(美国Pharmacia公司);反相柱色谱硅胶12 nm S-50 μm (日本株式会社YMC);柱色谱硅胶(200~300 目)和 GF254 ( 100 mm × 100 mm) 硅胶板(青岛海洋化工厂);分析级乙醇、乙酸乙酯、甲醇、二氯甲烷(上海泰坦科技股份有限公司);色谱级甲醇、乙腈(上海泰坦科技股份有限公司);屈臣氏蒸馏水(广州屈臣氏食品饮料有限公司);发酵用水为去离子水;显色剂为香草醛-硫酸显色剂(12 g 香草醛,250 ml 乙醇,200 ml 浓硫酸,500 ml 蒸馏水)。
-
本实验所用菌株为2020年从产自福建南靖的一株金线莲(Anectochilus roxburhii)中分离得到。采样植株经福建中医药大学吴岩斌副研究员鉴定为金线莲,内生真菌经形态学鉴定为曲霉菌属Aspergillus sp.,编号为J218,现保存于海军军医大学中药鉴定学教研室。
-
将保存的金线莲内生真菌菌株Aspergillus sp. J218接种至经高压灭菌的PDA平板培养基上,于培养箱活化培养7 d,用灭菌的打孔器打孔取菌饼置于装有250 g大米培养基的1000 ml锥形瓶中,共40瓶,常温发酵30 d。
-
发酵产物每瓶用500 ml 95%乙醇超声提取二次,每次45min, 过滤,减压浓缩至无醇味,将发酵提取物用石油醚(1∶1.5)脱脂2次,然后用乙酸乙酯(1∶2)萃取3次,合并乙酸乙酯部分萃取液,减压浓缩得到褐色浸膏24.7 g。将24.7 g浸膏由适量甲醇溶解,上样于Sephadex LH-20柱色谱,以80%甲醇进行洗脱,得到5个组分(Fr. 1~5)。
Fr.2析出淡黄色结晶,经300~400目硅胶色谱以二氯甲烷:甲醇(30∶1)洗脱得到化合物1(49.2 mg)。Fr.3部分经200目硅胶柱色谱,以二氯甲烷:甲醇(200∶1→7∶1)进行梯度洗脱,同时用TLC进行点板检测,合并得到5个组分Fr.3-1~3-5。Fr.3-4经300~400目硅胶色谱以二氯甲烷:甲醇(75∶1)洗脱得到化合物2(5 mg);Fr.3-5经Sephadex LH-20凝胶柱(80%甲醇洗脱)和300~400目硅胶色谱以石油醚:乙酸乙酯:甲醇(5∶10∶0.5)洗脱得到化合物3(22.6 mg);Fr.3-2 经200目硅胶柱色谱(二氯甲烷,二氯甲烷:甲醇=1000∶1→100∶1)得到5个亚组分Fr.3-2-1~3-2-5。Fr.3-2-5经300-400目硅胶色谱以二氯甲烷:甲醇(105∶1)洗脱得到化合物4(27.7 mg);Fr.3-2-4以65%甲醇-0.05%甲酸水为流动相,经Agilent 1200 Series 半制备型液相色谱,色谱柱为Eclipse XDB-C18,流速为2 ml/min,收集22 min馏分减压浓缩得到化合物5(49.3 mg);Fr.3-2-2经300-400目硅胶色谱以二氯甲烷:甲醇(135∶1)洗脱得到化合物6(10.7 mg)和化合物7(6.8 mg);Fr.3-2-2经300~400目硅胶色谱以二氯甲烷:甲醇(120∶1)洗脱得到化合物8(15.7 mg)和化合物9(11.5 mg)。Fr.4部分经200目硅胶柱色谱,以二氯甲烷:甲醇(800∶1→80∶1)进行梯度洗脱,得到化合物10(2.3 mg)。
-
化合物1:黄色粉末。分子式:C24H22O8, ESI-MS m/z: 439 [M+H]+。1H NMR (600 MHz, Chloroform-d) δH: 6.73 (s, 1H, H-6, 6′), 5.51 (s, 1H, H-3, 3′), 3.93 (s, 3H, 4,4′-OCH3), 3.80 (s, 3H, 5, 5′- OCH3), 2.70 (s, 3H, 7, 7′-CH3). 13C NMR (150 MHz, Chloroform-d) δC:169.88 (C-4, 4′), 163.12 (C-2, 2′), 159.57 (C-7, 7′), 153.49 (C-8a, 8a′), 138.52 (C-5, 5′), 111.43 (C-6, 6′), 108.45 (C-8, 8′), 107.47 (C-4a, 4a′), 87.72 (3, 3′-CH3), 56.11 (7, 7′- OCH3), 55.89 (4, 4′- OCH3), 24.06 (5, 5′-CH3) [10], 以上波谱数据与文献对照一致,确定化合物1为kotanin。
化合物2:黄色粉末。分子式:C16H14O5, ESI-MS m/z: 285 [M-H]−。1H NMR (600 MHz, Chloroform-d) δH: 12.81 (s, 1H, 5-OH), 6.88 (s, 1H, H-6), 6.60 (d, J = 2.2 Hz, 1H, H-7), 6.41 (d, J = 2.2 Hz, 1H, H-9), 6.29 (s, 1H, H-3), 3.99 (s, 3H, 10-OCH3), 3.94 (s, 3H, 8-OCH3), 2.51 (s, 3H, 2-CH3); 13C NMR (150 MHz, Chloroform-d) δC: 182.83 (C-4), 166.54 (C-2), 161.43 (C-8), 159.05 (C-10), 156.61 (C-5), 155.79 (C-11), 141.18 (C-13), 110.21 (C-3), 108.84 (C-14), 105.77 (C-6), 104.88 (C-12), 97.93 (C-7), 96.98 (C-9), 55.85 (10- OCH3), 55.44 (8- OCH3), 20.46 (2-CH3) [11], 以上波谱数据与文献对照一致,确定化合物2为flavasperone。
化合物3:黄色粉末。分子式:C32H30O12, ESI-MS m/z: 607 [M+H]+。1H NMR (600 MHz, Chloroform-d) δH: 14.53 (s, 1H, 5′-OH), 14.17 (s, 1H, 5-OH), 6.88~6.78 (m, 1H, H-9), 6.69 (d, J = 17.8 Hz, 1H, , H-10), 6.37 (d, J = 2.2 Hz, 1H, H-7′), 6.13 (dd, J = 8.5, 2.3 Hz, 1H, H-9′), 4.00 (s, 3H, 6′-OCH3), 3.79 (d, J = 9.8 Hz, 3H, 8-OCH3), 3.68~3.61 (m, 3H, 8′-OCH3), 3.38 (d, J = 3.1 Hz, 3H, 6-OCH3), 3.07~2.99 (m, 2H, 3-H2), 2.98~2.87 (m, 2H, 3′-H2), 1.79 (d, J = 6.0 Hz, 3H, 2-CH3), 1.48 (d, J = 3.8 Hz, 3H, 2′-CH3); 13C NMR (150 MHz, Chloroform-d) δC: 197.60 (C-4′), 196.89 (C-4), 164.85 (C-8′), 163.81 (C-8), 162.15 (C-6′), 161.81 (C-6), 161.01 (C-5′), 158.18 (C-5), 153.19 (C-10a), 151.34 (C-10a′), 142.66 (C-9a), 142.39 (C-9a′), 117.81 (C-7), 110.55 (C-5a), 107.73 (C-5a′), 106.44 (C-10′), 103.79 (C-4a), 103.58 (C-4a′), 102.77 (C-9), 102.24 (C-10), 100.21 (C-2), 100.11 (C-2′), 97.37 (C-7′), 96.21 (C-9′), 61.58 (C-6), 56.17 (C-6′), 55.89 (C-8), 55.16 (C-8′), 47.33 (C-3), 46.92 (C-3′), 29.24 (2′-CH3), 28.69 (2-CH3) [12, 13], 以上波谱数据与文献对照一致,确定化合物3为aurasperone B。
化合物4:微黄色粉末。分子式:C32H28O11, ESI-MS m/z: 587 [M-H]−。1H NMR (600 MHz, Chloroform-d) δH: 14.77 (s, 1H, 5-OH), 14.52 (s, 1H, 5′-OH), 7.12 (s, 1H, H-10), 6.97 (s, 1H, H-9), 6.36 (d, J = 2.2 Hz, 1H, H-7′), 6.13 (d, J = 2.3 Hz, 1H, H-9′), 6.04 (s, 1H, H-3), 4.00 (s, 3H, 6′-OCH3), 3.82 (s, 3H, 8-OCH3), 3.64 (s, 3H, 8′-OCH3), 3.42 (s, 3H, 6-OCH3), 2.92 (d, J = 8.7 Hz, 2H, 3′-H2), 2.40 (s, 3H, 2-CH3), 1.48 (s, 3H, 2′-CH3); 13C NMR (150 MHz, Chloroform-d) δC: 197.49 (C-4′), 184.46 (C-4), 167.68 (C-2), 164.96 (C-8′), 162.18 (C-8), 161.89 (C-6′), 161.86 (C-6), 160.22 (C-5), 157.40 (C-5′), 153.18 (C-10a), 151.30 (C-10a′), 142.62 (C-9a), 140.21 (C-9a′), 118.64 (C-7), 111.39 (C-5a), 107.83 (C-5a′), 107.36 (C-3), 106.62 (C-10′), 104.72 (C-4a), 103.84 (C-4a′), 101.75 (C-9), 101.18 (C-10), 100.23 (C-2′), 97.44 (C-7′), 96.23 (C-9′), 61.78 (6-OCH3), 56.19 (6′-OCH3), 55.90 (8-OCH3), 55.15 (8′-OCH3), 46.88 (C-3′), 28.86 (2′-CH3), 20.74 (2-CH3) [14, 15], 以上波谱数据与文献对照一致,确定化合物4为fonsecinone B。
化合物5:淡黄色粉末。分子式:C32H28O11, ESI-MS m/z: 589 [M+H]+。 1H NMR (600 MHz, Chloroform-d) δH: 14.94 (brs, 1H), 14.17 (d, J = 4.3 Hz, 1H), 7.00 (s, 1H, H-10), 6.86 (d, J = 3.7 Hz, 1H, H-9), 6.73 (d, J = 3.3 Hz, 1H, H-7′), 6.43 (d, J = 2.2 Hz, 1H, H-9′), 6.24 (d, J = 2.3 Hz, 1H, H-3′), 6.00 (t, J = 15.3 Hz, 1H, 2-OH), 4.04 (s, 3H, 6′-OCH3), 3.78 (s, 3H, 8-OCH3), 3.65 (d, J = 1.4 Hz, 3H, 8′-OCH3), 3.44 (s, 3H, 6-OCH3), 3.13~2.94 (m, 2H, 3-H2), 2.21~2.10 (m, 3H, 2′-CH3), 1.82 (s, 3H, 2-CH3); 13C NMR (150 MHz, Chloroform-d) δC: 196.59 (C-4), 184.58 (C-4′), 167.56 (C-2′), 164.06 (C-8), 162.63 (C-8′), 161.43 (C-6′), 161.04 (C-6), 160.96 (C-5), 159.43 (C-5′), 153.32 (C-10a′), 150.80 (C-10a), 142.74 (C-9a), 140.71 (C-9a′), 116.80 (C-7), 110.70 (C-5a′), 108.57 (C-5a), 107.20 (C-3′), 105.17 (C-10), 104.25 (C-4a′), 103.51 (C-4a), 102.67 (C-9), 101.85 (C-10′), 100.12 (C-2), 96.96 (C-7′), 96.49 (C-9′), 61.87 (6′-OCH3), 56.19 (6-OCH3), 55.91 (8′-OCH3), 55.18 (8-OCH3), 47.35 (C-3), 28.68 (2-CH3), 20.68 (2′-CH3) [12, 14], 以上波谱数据与文献对照一致,确定化合物5为fonsecinone D。
化合物6:黄色粉末。分子式:C13H11NO3, ESI-MS m/z: 230 [M+H]+。1H NMR (600 MHz, Chloroform-d) δH: 9.11 (s, 1H, 4-OH), 8.75 (s, 1H, H-2), 7.42~7.37 (m, 2H, H-9, 13), 7.37~7.31 (m, 1H, H-11), 7.26 (dt, J = 6.2, 1.4 Hz, 2H, H-10, 12), 6.27 (s, 1H, H-5), 6.03 (s, 1H, 3-OH), 3.91 (s, 2H, 7-H2); 13C NMR (150 MHz, Chloroform-d) δC: 178.08 (C-3), 168.85 (C-6a), 164.15 (C-4), 162.07 (C-2), 133.79 (C-8), 129.12 (C-9,13), 127.83 (C-10,12), 119.26 (C-3a), 116.06 (C-5), 39.60 (C-7) [16], 以上波谱数据与文献对照一致,确定化合物6为tensidol A。
化合物7:黄色粉末。分子式:C32H26O10, ESI-MS m/z: 571 [M+H]+。1H NMR (600 MHz, Chloroform-d) δH: 15.22 (brs, 1H,5′-OH), 12.81 (brs, 1H, 5-OH), 7.08 (s, 1H, H-6), 7.00 (s, 1H, H-7), 6.46 (d, J = 2.1 Hz, 1H, H-7′), 6.37 (s, 1H, H-3), 6.22 (d, J = 2.1 Hz, 1H, H-9′), 6.04 (s, 1H, H-3′), 4.06 (s, 3H, 6′-OCH3), 3.81 (s, 3H, 8-OCH3), 3.65 (s, 3H, 8′-OCH3), 3.46 (s, 3H, 10-OCH3), 2.52 (s, 3H, 2-CH3), 2.15 (s, 3H, 2′-CH3); 13C NMR (151 MHz, Chloroform-d) δC: 184.59 (C-4′), 183.01 (C-4), 167.48 (C-2), 166.87 (C-2′), 162.83 (C-5′), 161.61 (C-8′), 161.16 (C-6′), 160.07 (C-8), 156.96 (C-10), 156.69 (C-5), 155.15 (C-11), 150.86 (C-10a′), 140.82 (C-6a), 140.67 (C-9a′), 117.16 (C-9), 110.68 (C-3), 108.65(C-5a′), 108.01 (C-10a), 107.37 (C-3′), 106.07 (C-6), 105.05 (C-10′), 104.28 (C-4a′), 101.57 (C-7), 97.03(C-7′), 96.36 (C-9′), 61.21 (10-OCH3), 56.23 (6′-OCH3), 55.98 (8-OCH3), 55.18 (8′-OCH3), 20.67 (2′-CH3), 20.55 (2-CH3) [15, 17], 以上波谱数据与文献对照一致,确定化合物7为fonsecinone A。
化合物8:黄色粉末。分子式:C32H28O11, ESI-MS m/z: 588 [M+H]+。1H NMR (600 MHz, Chloroform-d) δH: 14.55 (s, 1H, 5′-OH), 12.79 (s, 1H, 5-OH), 7.07 (s, 1H, H-7), 7.02 (s, 1H, H-6), 6.41 (d, J = 2.2 Hz, 1H, H-7′), 6.37~6.35 (m, 1H, H-3), 6.17 (d, J = 2.2 Hz, 1H, H-9′), 4.04 (s, 3H, 6′-OCH3), 3.86 (s, 3H, 8-OCH3), 3.67 (s, 3H, 8′-OCH3), 3.42 (s, 3H, 6-OCH3), 2.97 (d, J = 18.4 Hz, 2H, 3′-H2), 2.51 (s, 3H, 2-CH3), 1.49 (s, 3H, 2′-CH3); 13C NMR (150 MHz, Chloroform-d) δC: 197.18 (C-4′),182.70 (C-4), 166.66 (C-2), 165.11 (C-8′), 162.45 (C-8), 161.91 (C-6′), 160.06 (C-10), 156.55 (C-5), 155.61 (C-5′), 154.98 (C-11), 151.13 (C-10a′), 142.53 (C-6a), 140.45 (C-9a′), 118.17 (C-9), 110.74 (C-10′), 109.4 (C-10a), 107.93 (C-5a′), 107.90 (C-3), 106.58 (C-10), 106.05 (C-4a), 103.71 (C-4a′), 102.01 (C-6), 100.22 (C-2′), 97.31 (C-7′), 96.49 (C-9′), 61.13 (10-OCH3), 56.20 (6′-OCH3), 55.98 (8-OCH3), 55.21 (8′-OCH3), 46.65 (C-3′), 29.16 (2′-CH3), 20.51 (2-CH3) [15], 以上波谱数据与文献对照一致,确定化合物8为fonsecinone C。
化合物9:黄色粉末。分子式:C32H26O10, ESI-MS m/z: 571 [M+H]+。1H NMR (600 MHz, Chloroform-d) δH: 15.20 (s, 1H, 5′-OH), 14.79 (s, 1H, 5-OH), 7.11 (s, 1H, H-10), 6.93 (s, 1H, H-9), 6.37 (d, J = 2.2 Hz, 1H, H-7′), 6.17 (d, J = 2.2 Hz, 1H, H-9′), 6.01 (d, J = 0.8 Hz, 1H, H-3), 5.94 (d, J = 0.8 Hz, 1H, H-3′), 3.98 (s, 3H, 6′-OCH3), 3.74 (s, 3H, 8-OCH3), 3.58 (s, 3H, 8′-OCH3), 3.42 (s, 3H, 6-OCH3), 2.37 (d, J = 0.7 Hz, 3H, 2-CH3), 2.07 (d, J = 0.7 Hz, 3H, 2′-CH3); 13C NMR (150 MHz, Chloroform-d) δC: 184.60 (C-4′), 184.43 (C-4), 167.62 (C-2), 167.54 (C-2′), 162.72 (C-5′), 162.01 (C-5), 161.41 (C-8′), 161.07(C-6′), 160.20 (C-8), 158.61 (C-6), 153.37 (C-10a), 150.84 (C-10a′), 140.70 (C-9a), 140.55 (C-9a′), 117.63 (C-7), 111.45 (C-5a), 108.59 (C-5a′), 107.44 (C-3), 107.26 (C-3′), 105.17 (C-10′), 104.73 (C-4a), 104.27 (C-4a′), 101.35 (C-9), 101.21 (C-10), 96.88 (C-7′), 96.53 (C-9′), 62.01 (6-OCH3), 56.21 (6′-OCH3), 55.91 (8-OCH3), 55.13 (8′-OCH3), 20.75 (2-CH3), 20.68 (2′-CH3) [11, 17], 以上波谱数据与文献对照一致,确定化合物9为aurasperone A。
化合物10:黄色粉末。分子式:C31H26O11, ESI-MS m/z: 575 [M+H]+。1H NMR (600 MHz, Chloroform-d) δH: 15.11 (s, 1H, 5′-OH), 14.28 (s, 1H, 5-OH), 7.05 (s, 1H, H-9), 6.68 (d, J = 2.4, 1H, H-7′), 6.28 (d, J = 2.4, 1H, H-9′), 5.95 (s, 1H, H-10), 5.91 (s, 1H, H-3′), 3.86 (s, 3H, 6′-OCH3), 3.69 (s, 3H, 8′-OCH3), 3.42 (s, 3H, 6-OCH3), 3.00 (dd, J = 16.9, 3.8 Hz, 2H, 3-H2), 2.14 (s, 3H, 2′-CH3), 1.28 (s, 3H, 2-CH3); 13C NMR (150 MHz, Chloroform-d) δC: 196.48 (C-4), 184.14 (C-4′), 168.21 (C-2′), 164.48 (C-5), 163.38 (C-5′), 162.22 (C-8′), 160.92 (C-6′), 159.81 (C-8), 157.63 (C-6), 152.99 (C-10a), 151.98 (C-10a′), 142.79 (C-9a), 140.72 (C-9a′), 115.05 (C-7), 110.59 (C-5a), 108.45 (C-5a′), 107.32 (C-3′), 106.50 (C-9), 106.48 (C-10′), 104.14 (C-4a′), 103.30 (C-4a), 102.31 (C-10), 100.02 (C-2), 97.21 (C-9′), 95.94 (C-7′), 62.03 (6-OCH3), 55.86 (6′-OCH3), 55.31 (8′-OCH3), 47.19 (C-3), 28.80 (2-CH3), 20.72 (2′-CH3) [18], 以上波谱数据与文献对照一致,确定化合物10为aurasperone F。
-
本研究选取从金线莲植株中分离得到的一株内生真菌Aspergillus sp. J218为研究对象,从其大米固体发酵产物中共分离鉴定出10个化合物。其中化合物3、4、5、7、8、9、10均为二聚萘并吡喃酮类化合物,化合物1为香豆素类化合物二聚体。据报道,萘并吡喃酮类化合物的单体及二聚体类广泛分布于曲霉属和镰刀属真菌的次级代谢产物中,二聚萘并吡喃酮类化合物对多种致病菌,如绿脓杆菌、克鲁斯假丝酵母、幽门螺杆菌等,有中等抑菌活性[19],对多种癌细胞如MDA-MB-231、PANC-1、A549等表现出一定抑制活性[20-21]。研究表明,化合物7和9对幽门螺杆菌具有中等抑制活性[22];化合物8对 A549、HL-60 和 MGC-803细胞具有不用程度抑制作用[23];化合物6能够增强咪康唑对白色念珠菌的抑制活性[24];化合物1对枯草芽孢杆菌和尖孢镰刀菌具有一定的抑制作用[25]。由此可见,从Aspergillus sp. J218中分离鉴定的多数化合物对病原微生物具有一定的抑制作用,提示该菌株在提升宿主植物抗病性方面具有潜在的应用价值;所鉴定化学结构类型提示该菌株含有丰富的二聚化合成酶,但相关研究较少。Frandsen等推测二聚萘并吡喃酮类化合物是由乙酰辅酶A和丙二酰辅酶A分子经过一系列缩合、甲基化和氧化还原反应形成萘并吡喃酮单体,再由一种假定的漆酶Gip1将单体羟基上一个电子移除,然后通过电子重排形成碳活化单体,自身或相互偶联形成二聚体[26];徐丹等[27]发现二聚萘并吡喃酮化合物ustilaginoidins中C-2和C-3双键的还原是由一种磷脂甲基转移酶(UsgR)催化形成,这种还原酶对线性萘并-γ-吡喃酮单体的还原具有特异性,但对二聚体则无特异性,而漆酶(UsgL)对各种单体的阻转异构选择性偶联导致了ustilaginoidins系列衍生物的结构多样性。上述研究为该菌株后续探索该类芳香族聚酮化合物二聚化的生物合成途径提供了一条线索。
Secondary metabolites of endophytic fungus Aspergillus sp. J218 from Anectochilus roxburhii
doi: 10.12206/j.issn.2097-2024.202208068
- Received Date: 2022-08-16
- Rev Recd Date: 2023-01-09
- Publish Date: 2023-07-25
-
Key words:
- Aspergillus sp. J218 /
- Anectochilus roxburhii /
- endophytic fungi /
- secondary metabolites /
- dimeric naphthopyrones
Abstract:
| Citation: | HE Xuhui, LI Xiang-ang, CHEN Meiyan, WANG Yiding, WU Rui, ZHENG Chengjian. Secondary metabolites of endophytic fungus Aspergillus sp. J218 from Anectochilus roxburhii[J]. Journal of Pharmaceutical Practice and Service, 2023, 41(7): 411-415, 442. doi: 10.12206/j.issn.2097-2024.202208068 |








 DownLoad:
DownLoad: