-
栀子是茜草科植物栀子(Gardenia jasminoides Ellis)的干燥成熟果实[1],主要产地为江西省抚州市、河南省唐河县等,中医药应用历史悠久,是一种药食两用资源。其具有清热祛火、缓解心烦、消肿止痛、化痰止咳等功效,用于热病心烦、湿热黄疸、淋证涩痛、血热吐衄、目赤肿痛、火毒疮疡,外治扭挫伤痛[1]。近年来,国内外众多学者对栀子做了大量的研究,证明了栀子中含有环烯醚萜、单萜、二萜、三萜、黄酮类、有机酸和挥发性化合物等丰富的化学成分[2-4],具有抑制神经系统[5]、保肝[6-7]、抗氧化[8]、降血糖[9-11]、利胆[12]、镇痛[13-14]等药理作用,其中主要的有效成分为环烯醚萜苷类化合物。
目前对于环烯醚萜苷类化合物的富集纯化主要以大孔吸附树脂为主[15-18],本课题组前期建立了一种用栀子果实制备总环烯醚萜苷的方法,并获得了国家发明专利授权(ZL200510026144.5)。此制备工艺得率高,成本低廉,为环烯醚萜苷类成分的研究及探索栀子的药效物质基础提供技术支持,对栀子及其有效部位的全面质量评价至关重要。
栀子环烯醚萜苷类有效部位的质量控制指标与栀子药材相比,其重点在于薄层色谱鉴别、检查及含量测定,其中检查项包括水分、重金属及有害元素。中药在贮藏及保管的过程中,水分含量是影响药材质量及其性状的重要因素。水分较高可导致药材霉变或虫蛀,为微生物和害虫提供良好的生长繁殖条件,从而进一步影响其有效部位的质量。重金属污染是中药在生产、运输、贮藏过程中可能会出现的污染类型,而中药成分中的重金属污染主要包括铜、汞、砷、镉、铅等。现代科学表明,残留的重金属能够与人体中的酶蛋白牢固结合,对组织细胞的功能、结构产生不同程度的破坏。而用栀子果实制备总环烯醚萜苷时,有机化合物根据分子量大小可以经溶剂洗脱分开而达到除杂的目的,从而减少栀子有效部位中的重金属元素残留量。本研究采用大孔吸附树脂法制备栀子有效部位,选用电感耦合等离子体质谱法(ICP-MS)对栀子及其有效部位中残留的重金属元素进行测定,旨在为临床用药提供科学依据。
目前,总环烯醚萜苷的质量控制方法中仅以紫外分光光度法测定其含量[19],缺少针对总环烯醚萜苷整体的质量控制标准,故本研究在栀子药材的质量控制基础上建立了栀子有效部位的质量控制标准。
-
中药粉碎机(YF103-200g);KQ-800DE数控超声波清洗器(昆山市超声仪器有限公司);电热鼓风干燥箱(上海一恒科学仪器有限公司);SX2-4-10A箱式电阻炉(上海索域试验设备有限公司);ETHOS UP微波消解仪(意大利Milestone公司);Milli-Q Advantage超纯水仪(美国Merck Millipore公司);AP135W电子天平、ICPMS-2030电感耦合等离子体质谱仪、LC-10AT高效液相色谱仪[岛津企业管理(中国)有限公司]。
-
栀子药材购自安徽省亳州市;栀子有效部位(自制[15];栀子对照药材(批号:120986-201610,中国食品药品检定研究院);栀子对照品(批号:AF20062303,纯度98%,成都埃法生物科技有限公司);乙醇、乙酸乙酯、甲酸、甲醇(上海泰坦科技股份有限公司);丙酮、硫酸(国药集团化学试剂有限公司);浓硝酸(美国Fisher chemical公司);镉标准溶液(1000 μg/ml,批号:218025103,美国AccuStandard公司);铅标准溶液(1000 μg/ml,批号:221008-5)、砷标准溶液 (1000 μg/ml,批号:208030-6)、汞标准溶液(1000 μg/ml,批号:215022-7)、铜标准溶液(1000 μg/ml,批号:206020-5)均购自国家有色金属及电子材料分析测试中心;水为超纯水。
-
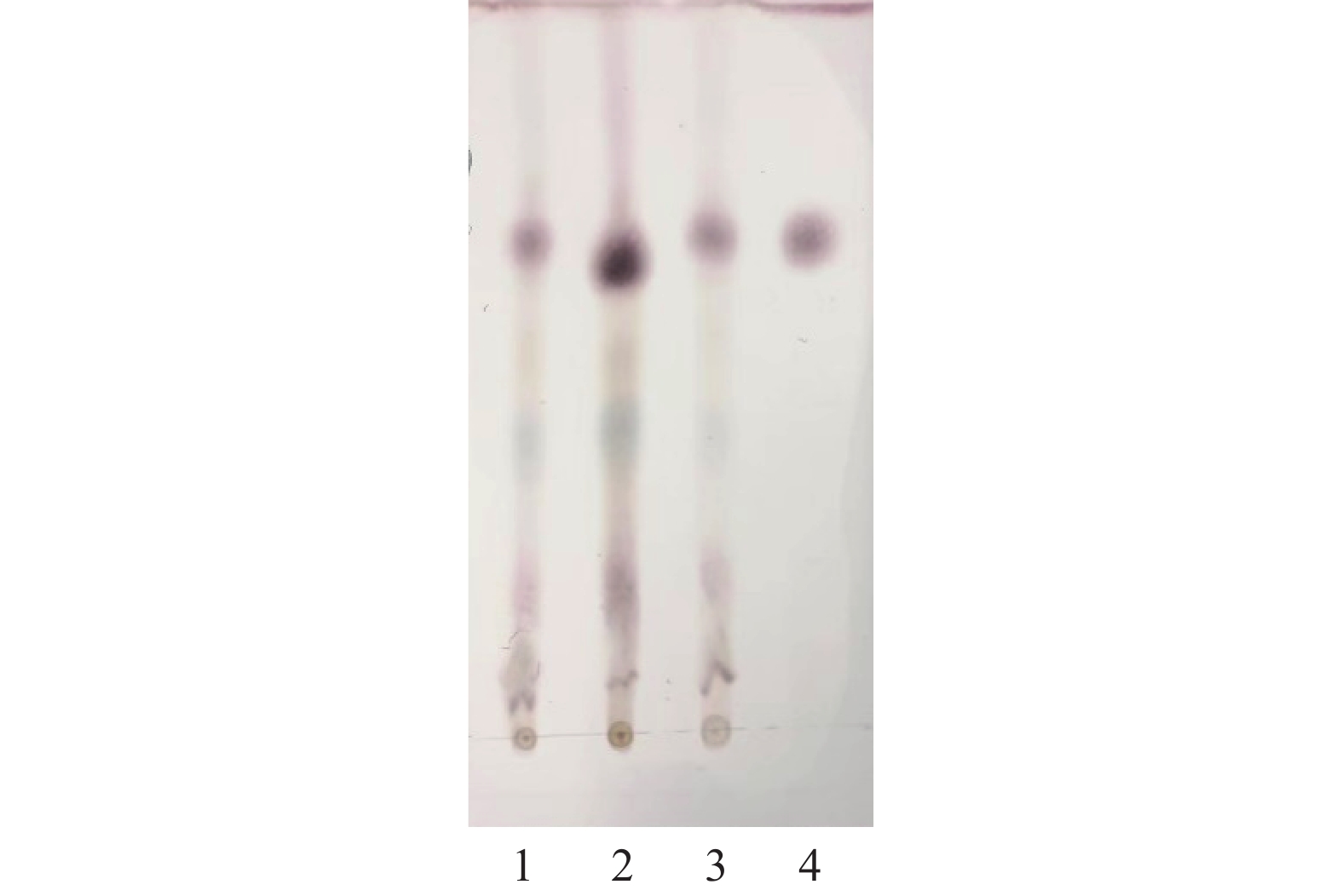
称取栀子粉末、栀子有效部位粉末和栀子对照药材粉末适量,参照《中国药典》(2020年版)一部栀子鉴别项下薄层色谱法,制备得到浓度分别为0.1004、0.1006、0.1012 g/ml的栀子和栀子有效部位供试品溶液以及栀子对照药材溶液。再取栀子苷对照品,以乙醇为溶剂制成4 mg/ml的对照品溶液。吸取上述溶液各3 μl分别点于同一硅胶G薄层板上,以乙酸乙酯-甲酸-丙酮-水(5∶1∶5∶1)为展开剂展开,取出,晾干;喷以10%硫酸乙醇溶液,于110 ℃加热使其显色,直至斑点显色清晰。栀子及其有效部位供试品色谱中,在与栀子对照品和栀子对照药材色谱相应的位置上显示相同的特征斑点(见图1)。
-
参照《中国药典》(2020年版)四部通则0832水分测定法第二法进行测定。测定栀子含水量为8.4%,符合《中国药典》(2020年版)一部栀子项下对水分的规定。栀子有效部位的含水量为3.2%。
-
参照《中国药典》(2020年版)四部通则2302总灰分测定法进行测定。测定总灰分为5.4%,符合《中国药典》(2020年版)一部栀子项下对总灰分的规定。
-
ICP-MS仪器主要参数设定见表1。
参数 参数设定 参数 参数设定 高频功率 1.20 kW 等离子体气流速 8.0 L/min 辅助气流速 1.10 L/min 载气流速 0.70 L/min 矩管类型 Mini矩管 雾化器 同心雾化器 雾化室 旋流 雾化室温度 5 ℃ 采样深度 5.0 mm 高频频率 27.12 MHz 碰撞气体 He 碰撞气流速 6 ml/min 池电压 −21 V 能量过滤器电压 7.0 V -
取栀子药材粗粉和栀子有效部位粉末各0.5 g,于60 ℃干燥2 h,加入硝酸8 ml,置于耐压耐高温微波消解罐中。0~10 min内温度从室温升至120 ℃,10~20 min从120 ℃升至180 ℃,于180 ℃保持20 min,最后从180 ℃降至60 ℃,按照此消解条件进行消解。消解完全后,待消解液冷却至60 ℃以下时,移出消解罐,将消解液转移至50 ml量瓶中,用适量水将消解罐洗涤3次,洗涤液合并于量瓶中,加入金标准溶液200 μl,以水为溶剂稀释至刻度,摇匀,即得。
-
精密吸取各标准储备液,以10%硝酸为溶剂配制成各元素相应质量浓度的混合系列溶液。其中,铅:0、1、5、10、20 ng/ml;镉:0、0.5、2.5、5、10 ng/ml;砷:0、1、5、10、20 ng/ml;铜:0、50、100、200、500 ng/ml;汞:0、0.2、0.5、1、2、5 ng/ml。以系列标准液浓度(X,ng/ml)为横坐标,元素响应值(Y)为纵坐标绘制标准曲线,得到各元素线性关系见表2。
元素 回归方程 r 线性范围
(ng/ml)方法检出限
(mg/kg)As Y=10.01048X+
0.2398920.9999 0~20 9.5×10−5 Cd Y=7.470893X+
0.1560680.9999 0~10 3.3×10−5 Cu Y=31.18023X−
44.787350.9999 0~500 3.5×10−4 Hg Y=3.455492X+
0.0173241.000 0~5 1.3×10−3 Pb Y=20.95865X+
3.0680300.9998 0~20 4.9×10−5 -
取含砷 1 ng/ml、镉 0.5 ng/ml、铜 50 g/ml、汞 0.2 ng/ml、铅1 ng/ml的混合标准溶液,在设定的仪器工作条件下重复测定5次,各测定元素的RSD值分别为0.53%、0.82%、0.32%、0.57%、0.40%,表明仪器精密度良好。
-
取栀子药材、栀子有效部位供试品粉末分别按 “2.4.2”项下方法操作,平行制备5份,在相同的仪器工作条件下进行分析,所测5种元素的RSD为0.30%~4.67%,表明该方法重复性良好。
-
各取上述栀子以及栀子有效部位的供试品溶液,分别于放置0、2、4、6、8 h后进行测定,计算两种供试品溶液中5种重金属元素的含量,结果各元素含量的RSD在0.15%~2.64%之间(n=5),表明两种供试品溶液中各测定元素在8 h内的稳定性良好。
-
精密称取已知含量的栀子以及栀子有效部位供试品各3份,分别精密加入各测定元素相应的对照品溶液适量,按“2.4.2”项下步骤操作,测定,计算各测定元素的回收率及RSD值,结果见表3。
测定元素 栀子 栀子有效部位 样品含量
(ng)加入量
(ng)测得量
(ng)平均回收率
(%)RSD
(%)样品含量
(ng)加入量
(ng)测得量
(ng)平均回收率
(%)RSD
(%)As 77.50 50 133.7 112 0.51 195.0 250 453.2 103 0.56 Cd 33.65 25 58.33 99 1.17 6.050 25 30.53 98 0.12 Cu 3740 5000 8650 98 0.00 1465 5000 6433 99 0.58 Hg 4.265 10 14.90 106 1.41 1.135 10 11.68 105 1.52 Pb 276.0 250 526.7 100 1.15 179.0 250 435.3 103 0.69 -
按“2.4.2”项下方法制备供试品溶液,按“2.4.1”项下工作条件测定两种样品,每份样品平行测定3次,结果见表4。
样品 As Cd Cu Hg Pb 栀子 0.156 0.0670 7.28 0.00844 0.553 栀子有效部位 0.390 0.0120 2.83 0.00179 0.361 -
色谱柱:Ultimate XB-C18(4.6 mm×150 mm,5 μm);流动相:乙腈-0.5%醋酸水(15:85);流速:1.0 ml/min;柱温:30 ℃;检测波长:238 nm;进样量:10 μl。
-
精密称取栀子苷对照品25.21 mg置于25 ml量瓶中,加甲醇制成含栀子苷1.008 mg/ml的溶液,精密量取0.3 ml至10 ml量瓶中,以甲醇为溶剂稀释至刻度,制成含栀子苷30.24 μg/ml的溶液。
-
分别精密称取栀子样品粉末(过四号筛)约0.1035g、栀子有效部位粉末13.36 mg,置于具塞锥形瓶中,精密加入25 ml甲醇,称定重量并记录,超声20 min后放冷,再次称定重量,用甲醇补足减失的重量,摇匀,滤过。精密量取续滤液10 ml,置于25 ml量瓶中,用甲醇加至刻度,摇匀,即得。
-
将空白对照溶液(甲醇)、栀子苷对照品溶液、栀子供试品溶液和栀子有效部位供试品溶液,按照上述色谱条件进样,得到色谱图(图2),栀子药材中其余成分及空白溶液对栀子苷的测定无影响。
-
将1.008 mg/ml的栀子苷对照品溶液用甲醇稀释制成322.6、161.3、80.64、40.32、20.16 μg/ml的系列溶液,分别吸取10 μl注入液相色谱仪,以对照品浓度(X,μg/ml)为横坐标,峰面积(Y)为纵坐标绘制标准曲线,得到标准曲线方程为Y=15860X+22543,r=0.9999,表明栀子苷在20.16~322.6 μg/ml的浓度范围内具有良好的线性关系。
-
吸取浓度为80.64 μg/ml的对照品溶液,在上述色谱条件下连续进样6次,结果栀子苷峰面积的RSD值为1.86%,表明仪器精密度良好。
-
取同一批栀子药材以及栀子有效部位粉末各6份,分别按上述供试品制备方法制备供试品溶液,按照上述色谱条件进样分析,记录峰面积。计算栀子药材及有效部位中栀子苷的含量,RSD分别为2.38%、2.60%,表明该方法重复性良好。
-
取栀子药材、栀子有效部位的供试品溶液,于制备后0、2、4、6、8 h分别按上述色谱条件进样测定。结果栀子苷峰面积的RSD分别为0.50%、0.81%。结果表明两种供试品溶液在室温下放置8 h稳定。
-
取栀子药材粉末约0.1 g,共6份,精密称定,分别置于具塞锥形瓶中,加入浓度为1 mg/ml的对照品溶液6 ml,精密加入甲醇19 ml,称定重量并记录,超声处理20 min,放冷,再次称定重量,用甲醇补足减失的重量,摇匀,滤过。精密量取续滤液10 ml,置25 ml量瓶中,加甲醇至刻度,摇匀,即得供试品溶液。按照上述色谱条件进样分析,结果见表5。
编号 药材粉
末量(g)原有量
(mg)加入量
(mg)测得量
(mg)回收率
(%)平均回
收率(%)RSD
(%)1 0.0964 4.708 6.006 10.65 98.9 99.1 2.18 2 0.0951 4.645 5.994 10.80 102.7 3 0.0933 4.557 6.132 10.45 96.1 4 0.1005 4.908 5.994 10.86 99.3 5 0.0931 4.547 6.090 10.60 99.4 6 0.1006 4.913 6.072 10.87 98.1 -
取栀子及栀子有效部位粉末按“2.5.3”项下方法分别配制供试品溶液,并按上述色谱条件进行测定,每份样品平行测定3次,计算得栀子及其有效部位中栀子苷含量分别为5.71%、34.2%,结果见表6。
种类 编号 称样量(mg) 含量(%) 平均(%) 栀子 1 100.7 5.76 5.71 2 100.5 5.87 3 100.5 5.51 栀子有效部位 1 13.60 33.5 34.2 2 13.40 34.7 3 13.57 34.4 -
栀子为传统中药,其有效部位是从栀子果实的乙醇提取物中制备得到的环烯醚萜苷类化合物,是后续制备中药复方制剂的常用成分,建立并规范栀子有效部位的质量标准,可以保证其质量,为临床疗效提供保障。
本实验对栀子有效部位进行薄层色谱鉴别、水分检查、重金属及有害元素检查、含量测定。水分、重金属及有害元素的含量直接影响到栀子有效部位的质量。环烯醚萜苷类成分易溶于水,水分含量过高时,在一定温度、酸度条件下,可能会产生颜色变化或沉淀。本文建立了电感耦合等离子体质谱法测定栀子有效部位中5种重金属元素的分析方法,通过线性、检出限、精密度、重复性、稳定性、加样回收率,对该方法进行考察,结果表明该方法准确度高、选择性好,可用于栀子有效部位中5种重金属元素的同时检测。
本实验测得栀子药材的含水量为8.4%,栀子有效部位的含水量为3.2%。栀子有效部位中镉、铜、汞、铅4种重金属元素的含量较栀子药材明显下降,而栀子苷的含量则显著提高。经分析,栀子果实制备得到总环烯醚萜苷粉末时,在减压浓缩干燥过程中水分蒸发导致栀子有效部位的含水量下降;栀子果实粗提物经大孔吸附树脂纯化时,先以少量水洗脱去除杂质,减少了环烯醚萜苷类成分的损失,再以低浓度乙醇洗脱有效成分,减少弱极性杂质的洗脱量,进而提高纯度,使得4种重金属元素含量降低,栀子苷含量提高。
本研究首次建立了栀子有效部位的质量控制方法,此方法重现性好、操作性强,能够有效控制栀子有效部位的质量,为全面控制中药复方制剂的质量和临床疗效提供依据。
Study on quality standard of Gardenia jasminoides and its effective parts
doi: 10.12206/j.issn.2097-2024.202210003
- Received Date: 2022-10-08
- Rev Recd Date: 2022-11-16
- Available Online: 2023-07-14
- Publish Date: 2023-02-25
-
Key words:
- gardenia /
- effective parts of Gardenia jasminoides /
- TLC /
- HPLC /
- geniposide /
- quality standards
Abstract:
| Citation: | GE Wen, GAO Congcong, LI Dongyang, CHEN Weidong, ZHOU Tingting. Study on quality standard of Gardenia jasminoides and its effective parts[J]. Journal of Pharmaceutical Practice and Service, 2023, 41(2): 113-118. doi: 10.12206/j.issn.2097-2024.202210003 |









 DownLoad:
DownLoad: