-
心力衰竭(HF) 是由心脏结构和/或功能异常引起,并由客观证据证实肺部或全身充血的一种心功能障碍所致的临床综合征[1],表现为体液潴留、呼吸困难、乏力等。心衰影响全球超过约6,400万人,总体患病率约为1.5%至4%,具有发病率高、死亡率高、生活质量差、治疗费用高等特点[2]。慢性心衰主要表现为射血分数降低的心力衰竭(EF≤40%, heart failure with reduced ejection fraction, HFrEF)[3]。2022 年美国心脏协会颁发指南,确定了HFrEF治疗的一线四联药物:血管紧张素转换酶抑制剂或血管紧张素受体脑啡肽酶抑制剂、β受体阻滞剂、盐皮质激素受体拮抗剂、钠-葡萄糖协同转运蛋白2抑制剂[4]。虽然目前治疗心衰的药物有很大进展,但心衰患者生存率依然面临严峻挑战,5 年生存率仅50%左右,与肿瘤生存率相仿[5]。因此,市场呼唤全新靶点的全新药物。
急性期蛋白ORM是本课题组自主发现的一个治疗心衰的潜在药物靶点,能够有效的改善小鼠的心功能,ORM尾静脉给药后显著增加心衰小鼠的射血分数,已经申请了专利(ZL201810565267.3)。然而,由于ORM是血液制品,价格昂贵,需注射给药,存在其药物开发的困难。本课题组获得了一个全新小分子化合物 HMS-01,该小分子化合物靶向上调ORM表达,有望进一步开发为具有治疗心衰作用的新靶点药物。为进一步了解HMS-01的药动学特点,本课题组采用液相色谱-串联质谱 (LC-MS/MS) 技术[6-7],研究建立灵敏、特异的测定血浆等生物样品中HMS-01 浓度的分析方法,并开展 HMS-01 在小鼠体内的药动学研究,为后续药物研究提供理论依据。
-
Agilent 1 290 InfinityⅡ液相色谱仪(AgilentTechnologies,美国)、4 000 Q-Trap 型串联质谱仪(AB Sciex,美国);低温高速离心机(Thermo,德国);Mettler XP56 百万分之一电子天平(梅特勒-托利多,瑞士); Millipore-Q 超纯去离子水净化仪(Millipore,美国);小型摇床和涡旋混合仪(IKA,德国)。
-
HMS-01(上海青玄生物科技有限公司,纯度:98.8%,批号:200502);内标罗红霉素(上海百灵威科技有限公司,纯度:98%,批号:LOC0T99);乙腈、甲醇(Sigma-aldrich,HPLC纯试剂);甲酸(纯度:LC-MS级)和甲酸铵(Aldrich-Fluka,纯度:LC-MS级);特丁基甲醚(Aldrich-Fluka,纯度:≥99.8%)。其它有机试剂为中国医药(集团)上海化学试剂公司市售分析纯。
-
健康C57BL/6J小鼠,雄性和雌性各123只,体重18~22g,由北京华阜康生物科技股份有限公司提供,合格证号:SCXK(京)2019-0008。自由饮水。
-
设置口服(p.o.)给药3个单剂量组(30、60及150 mg/kg体重)和一个静脉(i.v.)给药(6 mg/kg体重)单剂量组。C57BL/6J小鼠246只,随机分组,每组动物数为6,雌雄各半。在动物房适应5 d后,开始动物试验。给药HMS-01前后,在异氟烷麻醉状态下眼窝静脉窦采血,每只小鼠每次采血量约50~75 μl左右(2~3小滴血),置0.2 ml肝素化离心管中。采血时间点安排为:0、0.083(仅静脉组)、0.25、0.5、1、2、4、6、8、10及24 h。
-
小鼠血浆样品(40 µl)加入804 µl特丁基甲醚(含91 nmol/L 的IS内标)进行液液萃取。经振摇(1 600 r/min,5 min)和离心(14 800 r/min,5 min)后吸取600 µl上清液至1.5 ml离心管中,在N2气下吹干(30 ℃加热),用120 µl乙腈复溶。经振摇(1 600 r/min,5 min)和离心(14 800 r/min,5 min)后取50 µl用于进样分析。
-
色谱柱为 Gemini C18 (2.0 mm×50 mm, 5 μm),流动相:溶剂A:水(含 0.04‰甲酸+5 mmol/L甲酸铵),溶剂B:乙腈(含0.04‰甲酸),洗针液:乙腈。梯度洗脱,洗脱程序如下:0~2 min,90% A+10% B;2~6 min,5% A+95% B;6~8 min,90% A+10% B。流速:0.35 ml/min,柱温:25 ℃,进样量 5 μl,运行时间 8 min。
-
离子源参数:离子源为涡轮喷射,气帘气压力为20 psi,毛细管电压为 5.5 kV,辅助加热气温度为500 ℃,雾化气压力为 40 psi,辅助加热气压力为60 psi。各化合物MRM参数见表1。
化合物 去簇电压(V) 母离子(Da) 子离子(Da) 碰撞能(V) 碰撞室出口电压(V) HMS-01 106 772.5 214.2 39 16 罗红霉素 111 837.5 158.1 45 14 -
内标工作溶液配制:精密称取HMS-01对照品2.634mg,置于1.5ml EP管中,加0.675ml乙腈溶解,摇匀,得浓度为5mmol/L对照品储备液,−70 ℃存储。同时,精密称取4.366 mg的罗红霉素,置于1.5ml EP管中,加1.04 ml乙腈溶解,摇匀,得浓度为5mmol/L内标储备液,−70 ℃存储。
标准曲线溶液制备和质控溶液制备:以乙腈为溶剂,将HMS-01储备液稀释至浓度为20 µmol/L的工作液A,再采用逐级稀释法配制浓度为78.13、156.3、312.5、625、1 250、2 500、5 000、10 000 nmol/L的标准曲线工作液;再取各标准曲线工作液,以小鼠血浆为基质配制浓度为3.906 、7.81、15.6、31.25、62.5、125、250、500、1 000 nmol/L标准曲线溶液。随行质控溶液工作液HMS-01的低定量下限(LLOQ)为78.13nmol/L,其低、中、高浓度分别为160、1 600、16 000 nmol/L;质控样品LLOQ为3.906 nmol/L,低、中、高浓度分别为8、80、800 nmol/L。
-
选择性考察:取6个不同来源的小鼠空白血浆以及空白血浆配制的定量下限(LLOQ)样品按照“2.2”项下操作, 考察6个不同个体的空白小鼠血浆在对照品和内标保留时间处是否有干扰。
残留考察:运行定量上限(ULOQ)样品后,运行1个双盲样品,按 “2.2” 项下操作,至少考察3次。
-
以HMS-01与内标峰面积比分别对血浆样品中HMS-01的浓度进行线性回归(拟合方程Y=aX+b),并以浓度的倒数(1/X)为加权系数,求算回归方程。
-
定量下限血浆样品处理按照“2.2”项下操作, 进行6样本分析, 连续测定3批次, 并根据当前批次标准曲线计算每一样本的测得浓度, 计算该浓度的日内和日间精密度和准确度。
低、中、高浓度QC样品 (血浆浓度均分别为8.00、80.0和800 nmol/L) 处理按照“2.2 ”项下操作, 每个浓度进行6样本分析, 分别在第2日、3日计算实际浓度,测定批内、批间的精密度和准确度。
-
对6个批号的单个空白小鼠血浆样品进行处理,提取完成吹干后,在假定100%提取回收率的前提下,用含有低浓度(8 nmol/L)和高浓度(800 nmol/L)质控水平的HMS-01和内标(100 nmol/L)的复溶溶液复溶空白提取基质,测得的峰面积为Set1。
将包含低浓度(8 nmol/L)和高浓度(800 nmol/L)质控水平的HMS-01和内标(100 nmol/L)的复溶溶液,平行3份,测得的nmol/L峰面积为Set2。
基质效应(ME)= Set1/Set2×100%。
8、80、800 nmol/L的3个浓度的质控样品,按“2.2”项下方法处理后进样分析得待测物的峰面积为Set3,各浓度制备3个样品。
回收率(RE)= Set3/ average Set1×100%。
-
处理样品过程中的短期稳定性:小鼠血浆样品加特丁基甲醚3 h后HMS-01的稳定性以3个LQC(8 nmol/L)、3个HQC(800 nmol/L)作为一套稳定性样品。3个LQC和3个HQC样品各取2 µl,加内标4 µl和特丁基甲醚800 µl后,再加入38 µl小鼠空白血浆,在室温下放置3 h后,按“2.2”中描述的方法进行分析以考察其稳定性。
进样器上短期稳定性:小鼠血浆样品中HMS-01的进样盘放置稳定性以6个LQC(8 nmol/L)、6个HQC(800nmol/L)作为一套稳定性样品,在首次分析后继续储存在进样板上(进样器温度为8 ºC),放置11 h后用新绘标准曲线计算质控样品的浓度。
-
用小鼠血浆配制HMS-01浓度为50 µmol/L,按“2.2”项下方法进行样品前处理,乙腈复溶后得上清液备用。随后用乙腈(含91nmol/L的内标)溶液稀释该上清液,平行稀释6个样品,稀释倍数为100倍。
-
使用AB公司的Analyst软件输出原始图谱、浓度、准确度等数据。利用 EXCEL计算均值,标准偏差,变异系数等参数。HMS-01药动学参数用InnaPhase Kinetica2000TM软件(美国)用非房室模型分析处理。
-
采用Graphpad Prism 8 软件对数据进行处理和分析,结果以(均数±标准差)表示,采用t检验,P<0.05 认为差异有统计学意义,P<0.01 认为有极显著差异。
-
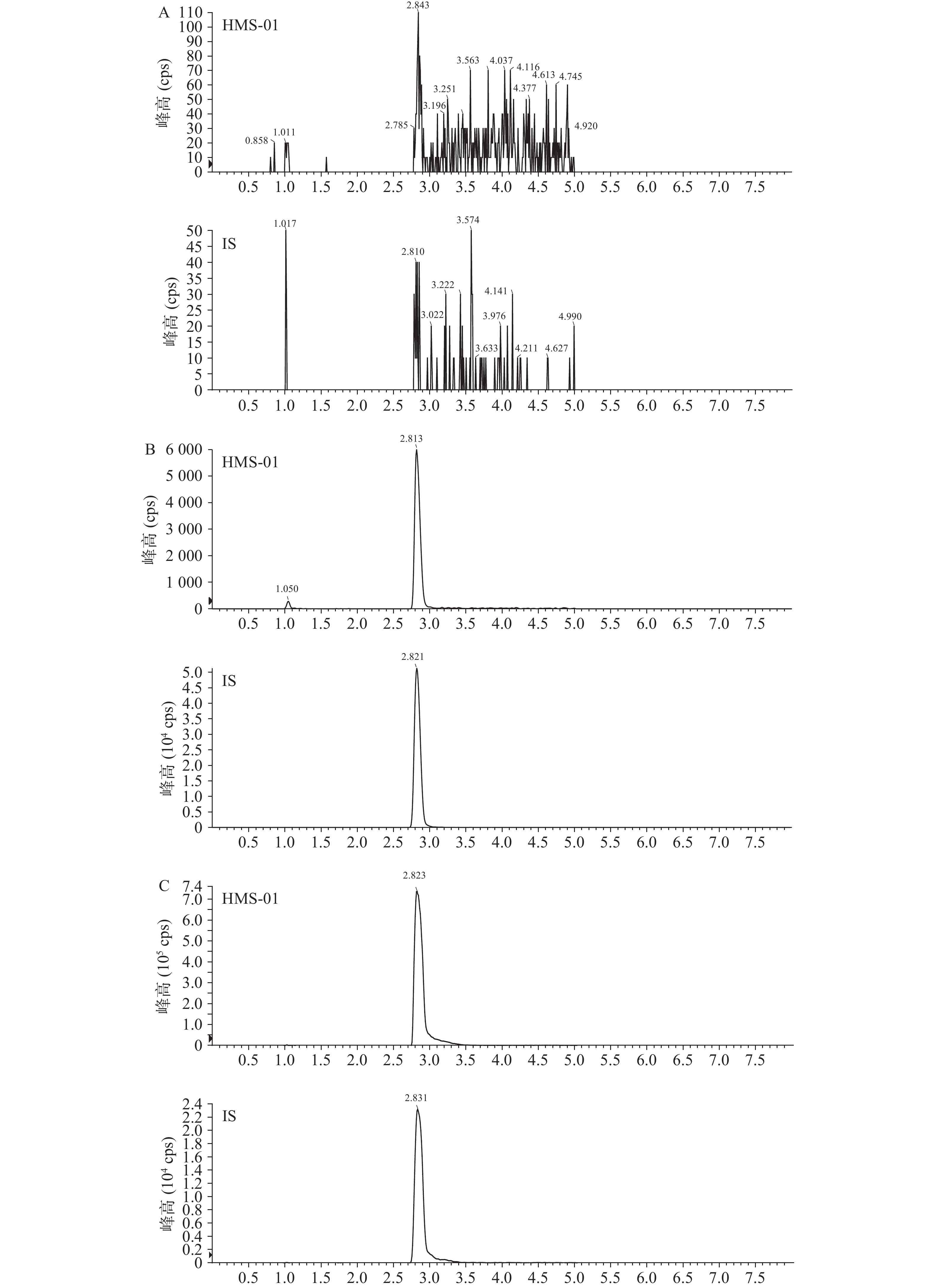
结果表明,空白小鼠血浆中的内源性物质不干扰HMS-01的测定。空白样品、LLOQ样品和给药(i.v.,HMS-01 2 030 nmol/L;i.v.,内标100 nmol/L)0.5 h血浆样品中成分色谱见图1。在本试验条件下,残留的各待测物响应值低于其定量下限各待测物响应值 20%,说明残留效应对血浆中各待测物测定的影响可以忽略不计。
-
测定小鼠血浆中HMS-01的线性范围为3.91~1 000 nmol/L。典型标准曲线回归方程为:Y = 0.021 5X + 0.007 56(r =0.999 5)。
-
定量下限血浆样品(HMS-01)为3.91 nmol/L的日内精密度为3%,准确度为105.9%;日间精密度为8.5%,准确度为115.9%。HMS-01低、中、高质控样品的精密度和准确度如表2所示。均符合生物样品测定相关要求[8]。
浓度(nmol/L) 日内精密度 日间精密度 实测浓度(nmol/L) 精密度(%) 准确度(%) 实测浓度(nmol/L) 精密度(%) 准确度(%) 8 7.838 2.04 97.98 7.783 4.92 97.29 80 76.93 0.89 96.16 85.24 9.76 106.55 800 710.3 1.41 88.75 821.5 9.96 102.69 -
内标归一化基质效应因子(待测物面积比/内标面积比)为0.92~1.12,内标归一化基质效应因子精密度CV均小于15%,说明建立的样品处理条件下基本无基质干扰。小鼠血浆中HMS-01提取回收率为 81.7%~95.1%,内标回收率为 81.4%~93.9%。提取回收率的各浓度精密度CV均小于15%,符合生物样品提取要求。
-
处理样品过程中的室温短期稳定性:各浓度的处理后样品平均实测浓度相对于理论浓度介于95.4%~109.1%。
处理后样品中HMS-01的稳定性:各浓度的处理后样品平均实测浓度相对于理论浓度介于87.4%~106.0%。
各浓度的处理后样品平均实测浓度相对于理论浓度均介于85%~115%,上述情况下待测物视为稳定。
稀释后准确度87.8%~111%,RSD 为8.5%表3,表明稀释可靠。
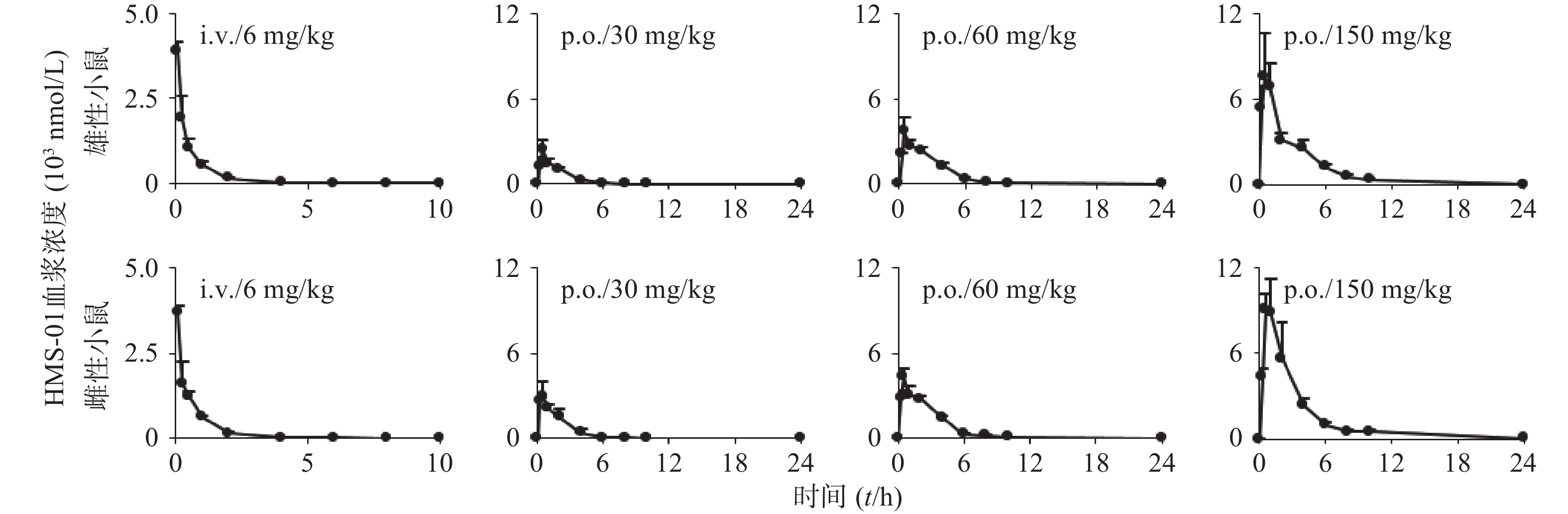
药代参数 i.v.(6 mg/kg) p.o(30 mg/kg) p.o(60 mg/kg) p.o(150 mg/kg) 雄小鼠组 雌小鼠组 雄小鼠组 雌小鼠组 雄小鼠组 雌小鼠组 雄小鼠组 雌小鼠组 Cmax(nmol) 3,910±254 3,700±184 2,420±728 3,207±704 3,770±939 4,337±536 7,933±2493 9,713±1,824 Tmax(h) − − 0.5±0 0.42±0.14 0.5±0 0.5±0 0.67±0.29 0.67±0.29 AUC0~24h (nmol·h) 1,919±79.1 1,914±142 4,566±656 6,233±733* 11,230±765 12,937±411* 23,320±4005 28,342±3300 AUC0~∞h(nmol·h) 1,923±78.1 1,917±142 4,638±654 6,301±722* 11,248±768 12,997±416* 23,346±3,999 28,375±3,289 t1/2 (h) 1.09±0.01 1.09±0.03 2.66±0.18 2.71±0.26 2.33±0.09 2.50±0.50 2.56±0.17 2.77±0.64 MRT (h) 2.07±1.35 1.86±0.75 2.82±0.26 2.41±0.21 2.96±0.15 3.09±0.49 3.42±0.30 3.32±0.16 CLtot,p
(L/(h·kg))2.75±0.10 2.85±0.31 − − − − − − VSS(L/kg) 5.62±3.48 5.17±1.64 − − − − − − F(%) − − 47.6±6.8 65.1±7.7* 58.5±4.0 67.6±2.1* 48.6±8.3 59.2±6.9 *P<0.05,与雄小鼠组比较 -
雌雄小鼠给药后不同时间点的血药浓度,相关平均时间-血药浓度曲线见图2。雌雄小鼠体内HMS-01暴露水平(AUC)分别与HMS-01剂量相关性分析结果见表3。小鼠药动学结果表明,HMS-01肠道吸收快,小鼠口服生物利用度中等(50%~70%)。HMS-01在小鼠体内的暴露水平 (AUC和cmax)随剂量的增加而增加,其中AUC随剂量的增加成线性相关。HMS-01静脉给药后,在小鼠体内的半衰期1 h左右;血浆清除率(CLtotal.p)为2.8 L/(h·kg),与小鼠肝血流量相当;表观分布容积(VSS)为5 L/kg,远大于小鼠总体液。雌雄小鼠30、60mg/kg经口服给药在AUC和F有显著差异(P<0.05),在cmax、 t1/2、CLtot,p、MRT、VSS等参数上接近,均无显著差异。
-
本文建立了快速、高效的LC-MS/MS法测定口服给药3个单剂量组和静脉给药单剂量组小鼠血浆中HMS-01浓度,经方法学验证,此方法的选择性、准确度、精密度、基质效应和稳定性均符合方法学要求,适用于小鼠体内血药浓度测定。本实验结果表明,HMS-01在小鼠体内的药动学过程有显著的性别差异,雌性小鼠鼠的生物利用度远高于雄性。考虑到心衰治疗需长期用药的现状,加上本实验中口服给药低、中、高剂量组显示HMS-01肠道吸收快,小鼠口服生物利用度尚可的特点,且HMS-01在小鼠体内的暴露水平(AUC和cmax)随剂量的增加而增加。因此,可考虑通过口服给药途径对HMS-01治疗小鼠心衰的进一步的体内研究提供依据。
Pharmacokinetic study of HMS-01 in mice
doi: 10.12206/j.issn.2097-2024.202211015
- Received Date: 2022-11-07
- Rev Recd Date: 2023-02-20
- Publish Date: 2023-03-25
-
Key words:
- HMS-01 /
- pharmacokinetics /
- mice /
- LC-MS/MS method
Abstract:
| Citation: | SHI Xiaofei, CHEN Yi, XIANG Kefa, JING Kai, GAO Yue, LIU Xia. Pharmacokinetic study of HMS-01 in mice[J]. Journal of Pharmaceutical Practice and Service, 2023, 41(3): 168-172, 191. doi: 10.12206/j.issn.2097-2024.202211015 |








 DownLoad:
DownLoad: