-
据世界卫生组织最新数据,截至2023年3月7日,全球已报告新型冠状病毒肺炎(COVID-19)确诊病例约7.6亿例,死亡超过686万例[1]。目前,抗病毒治疗可以抑制病毒复制,加快病毒清除,减轻炎症及免疫反应等,被认为是降低COVID-19重症率、住院率及死亡率的重要手段之一[2]。2021年12月,美国食品药品监督管理局批准奈玛特韦片/利托那韦片组合包装(Paxlovid)的紧急使用授权申请,使其成为治疗COVID-19的新型口服药物。2022年2月,国家药品监督管理局附条件批准Paxlovid进口注册[3],用于成人伴有进展为重症高风险因素的轻至中度COVID-19患者。国外多项研究已经证实,Paxlovid能显著降低COVID-19患者的病毒载量、全因住院率、重症率和死亡率等[4-5]。然而,目前罕见报道中国人群使用Paxlovid的疗效等相关数据。鉴于人种差异及病毒变异,开展此类研究十分必要。本研究旨在探索中国人群中Paxlovid对COVID-19患者早期预后不良的危险因素,并构建预测模型,以期为提高该类患者的救治效果提供参考。
-
回顾性分析2023年1月至2023年3月于闽南地区3家军队三甲医院(第九〇九医院、第九一〇医院和陆军第七十三集团军医院)使用Paxlovid的COVID-19住院患者。纳入标准:①COVID-19患者,诊断及临床分型符合《新型冠状病毒感染诊疗方案(试行第10版)》的标准[6];②年龄≥18岁;③用药时间≥3 d。排除标准:①未使用Paxlovid的患者;②Paxlovid停药28 d内失访的患者;③合并心、肺、肝、肾等重要器官严重损害者;④联合与Paxlovid存在严重相互作用的药物;⑤资料不全者等。本研究经牵头单位第九〇九医院伦理委员会批准通过。
-
收集患者的一般资料(性别、年龄、体重)、发病天数、Paxlovid疗程,合用药及辅助治疗情况、用药前的检查指标[血氧饱和度、核酸检测阈值循环数(CT值)、淋巴细胞计数、估算的肾小球滤过率(eGFR)、丙氨酸氨基转移酶(ALT)、天门冬氨酸氨基转移酶(AST)、三酰甘油(TG)、胆固醇(TC)、低密度脂蛋白(LDL-C)、高密度脂蛋白(HDL-C)、肌酸激酶(CK)、C反应蛋白(CRP)、降钙素原(PCT)、D-二聚体等]、合并疾病[高血压、动脉粥样硬化性心血管疾病(ASCVD)、慢性肺病等];结局指标[6]:Paxlovid停药28 d内若出现死亡或进展为重型、危重型COVID-19者则定义为早期预后不良;若Paxlovid停药28 d内出现COVID-19中型、轻型或痊愈者以及停药超过28 d出现死亡或进展为重型、危重型COVID-19者则定义为非早期预后不良。发病天数定义为患者首次出现临床症状或获得核酸检测阳性结果至第一次使用Paxlovid的日期。
-
运用SPSS 21.0软件进行统计分析,连续变量符合正态性分布的数据,采用(
$ \bar{x} $ ±s)表示,组间采用独立Student’s t检验;不符合正态性分布的数据采用中位数(四分位数间距)表示,组间采用Man-Whitney U检验;二分类变量以例数表示,采用χ2检验。单因素分析中P<0.05的变量,以向后LR法进入二元Logistic回归分析。采用受试者工作特征曲线(ROC)计算曲线下面积(AUC)评估模型的预测效能。P<0.05表示差异具有统计学意义。 -
2023年1月至2023年3月于闽南地区3家军队三甲医院使用Paxlovid治疗的COVID-19住院患者共129例,经筛选最终纳入92例进行分析。其中,男69例(75.00%),女23例(25.00%),平均年龄(76.26±15.81)岁,体重(62.86±10.69)kg,发病天数(8.60±5.94)d,核酸检测CT值(27.84±5.52)。
-
92例Paxlovid治疗的COVID-19患者中,早期预后不良者有31例(33.70%),其中,死亡11例(35.48%),危重型17例(54.84%),重型3例(9.68%)。
-
比较早期预后不良组与非早期预后不良组的患者一般资料、发病天数、检查指标、合并疾病、合用药及辅助治疗等情况,结果显示两组在发病天数、淋巴细胞计数、AST、CRP、PCT、D-二聚体等12项临床指标有相关性(P<0.05),见表1。
项目 非早期预后不良组(n=61) 早期预后不良组(n=31) 统计量 P值 年龄(岁,$ \bar{x} $±s) 77.16±16.47 74.42±14.52 0.785 0.434 性别[女,n(%)] 16(26.23) 7(22.58) 0.146 0.702 体重(m/kg,$ \bar{x} $±s) 63.99±10.09 60.63±11.63 1.430 0.156 发病天数(t/d,$ \bar{x} $±s) 7.72±5.46 12.48±6.56 −3.693 <0.001 血氧饱和度(%,$ \bar{x} $±s) 94.01±4.97 90.91±10.76 1.894 0.061 核酸检测CT值($ \bar{x} $±s) 28.31±5.64 26.90±5.24 1.158 0.250 淋巴细胞计数(×109/L,$ \bar{x} $±s) 0.98±0.59 0.62±0.44 2.992 0.040 eGFR[ml/(min·1.73 m2),$ \bar{x} $±s] 68.29±30.56 57.20±38.44 1.505 0.136 ALT[U/L,M(P25,P75)] 22.90(9.00,121.00) 23.00(6.00,237.20) −0.450 0.653a AST[U/L,M(P25,P75)] 31.00(13.50,88.00) 37.40(21.00,306.20) 2.747 0.006a TG(mmol/L,$ \bar{x} $±s) 1.36±0.87 1.50±0.76 −0.782 0.436 TC(mmol/L,$ \bar{x} $±s) 3.95±0.94 3.59±1.19 1.555 0.123 LDL-C(mmol/L,$ \bar{x} $±s) 2.19±0.69 2.01±0.97 0.998 0.321 HDL-C(mmol/L,$ \bar{x} $±s) 1.14±0.32 1.00±0.44 1.664 0.100 CK(U/L,$ \bar{x} $±s) 145.23±253.51 148.21±107.09 −0.062 0.950 CRP(mg/L,$ \bar{x} $±s) 52.41±46.71 118.72±82.74 −4.918 <0.001 PCT[ng/ml,M(P25,P75)] 0.07(0.01,27.60) 1.40(0.01,42.95) 4.366 <0.001a D−二聚体[μg/ml,M(P25,P75)] 0.79(0.19,11.39) 2.17(0.36,41.83) 4.254 <0.001a 用药前已使用其他抗新冠病毒药物治疗[n(%)] 1(1.64) 5(16.13) 7.079 0.008 用药前已行呼吸机辅助通气[n(%)] 10(16.39) 19(61.29) 19.194 <0.001 合并疾病 高血压[n(%)] 39(63.93) 20(64.52) 0.003 0.956 糖尿病[n(%)] 16(26.23) 14(45.16) 3.352 0.067 ASCVD[n(%)] 29(47.54) 15(48.39) 0.006 0.939 慢性肺病[n(%)] 19(31.15) 5(16.13) 2.404 0.121 治疗方案 Paxlovid疗程(t/d,$ \bar{x} $±s) 4.74±0.68 5.26±2.05 −1.805 0.074 联合免疫抑制剂[n(%)] 42(68.85) 29(93.55) 7.116 0.008 联合抗凝药[n(%)] 36(59.02) 29(93.55) 11.821 0.001 联合抗菌药物[n(%)] 46(75.41) 30(96.77) 6.530 0.011 联合俯卧位治疗[n(%)] 8(13.11) 9(29.03) 3.457 0.088 联合呼吸机辅助通气[n(%)] 11(18.03) 25(80.65) 33.831 <0.001 注:a表示Mann-Whitney U检验。 -
采用二元Logistic回归分析,结果显示其中发病天数、淋巴细胞计数、AST、CRP和联合呼吸机辅助通气等5项临床指标是Paxlovid早期预后不良的独立危险因素,见表2。上述5项独立危险因素构建Logistic模型方程,Logit(P)=−8.371+0.126X发病天数+2.019X淋巴细胞计数+0.023XAST+0.016XCRP+3.528X联合呼吸机辅助通气。采用H-L法(Hosmerand-Lemeshow test)对模型的拟合度进行检验,结果显示模型拟合良好(χ2值=10.480,P=0.233),模型的理论准确度为89.10%。
项目 回归系数B 标准误S.E 卡方值Waldχ2 自由度df 比值比OR 95%CI置信区间 P值 发病天数(t/d) 0.126 0.061 4.237 1 1.135 1.006~1.279 0.040 淋巴细胞计数 2.019 0.892 5.126 1 7.527 1.311~43.208 0.024 AST 0.023 0.009 6.578 1 1.023 1.005~1.041 0.010 CRP 0.016 0.007 5.744 1 1.016 1.003~1.029 0.017 联合呼吸机辅助通气 3.528 1.054 11.194 1 34.051 4.311~268.936 0.001 常量 −8.371 2.080 16.195 1 <0.001 -
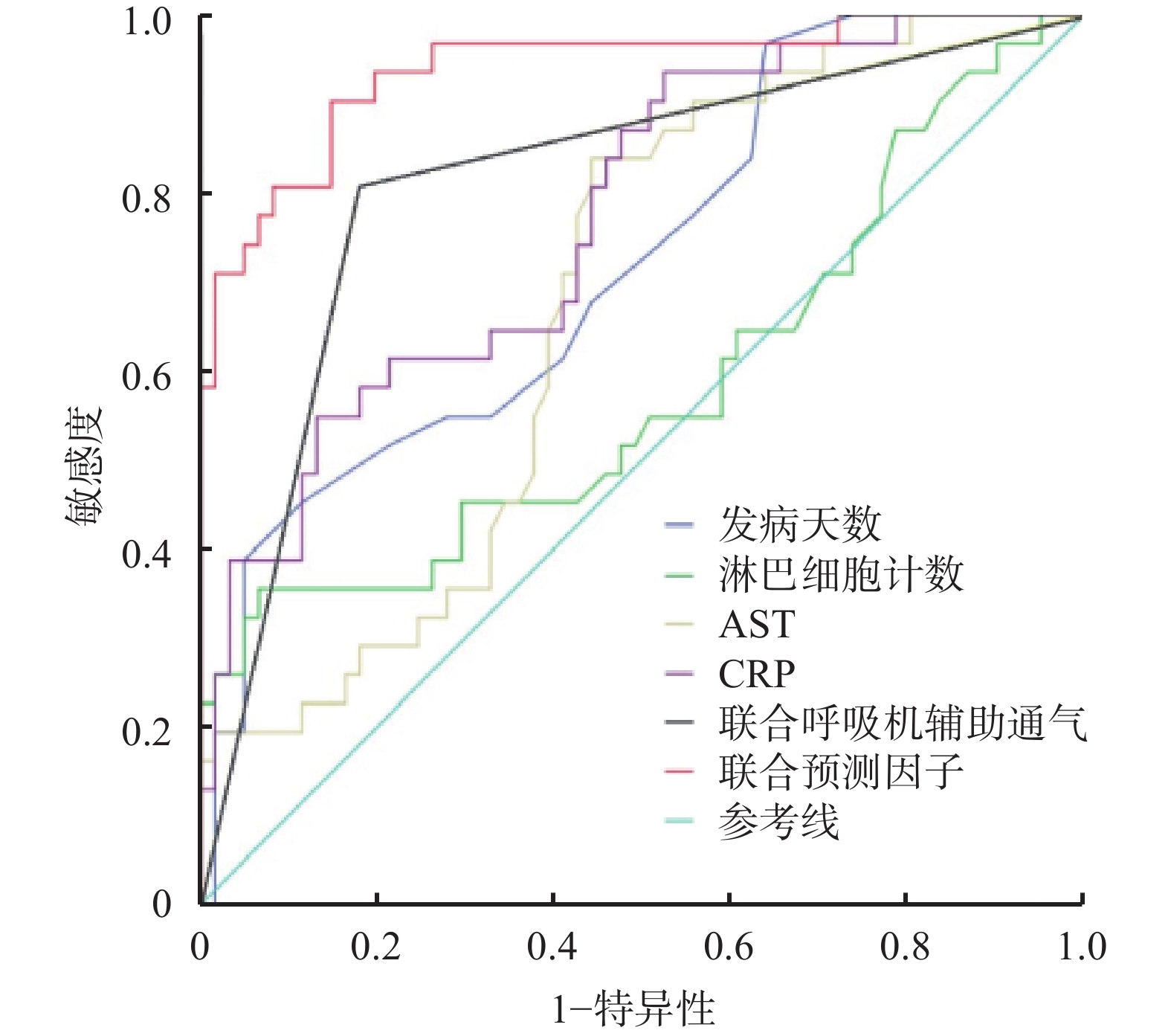
将上述回归方程转换后得出联合预测因子的计算公式,Y联合预测因子=7.875X发病天数+126.188X淋巴细胞计数+1.438XAST+XCRP+220.500X联合呼吸机辅助通气,计算92例患者的Y联合预测因子值,绘制ROC曲线,见图1。分别计算发病天数、淋巴细胞计数、AST、CRP和联合呼吸机辅助通气和联合预测因子AUC,其中联合预测因子AUC最大为0.939(P<0.001),预测价值最优。取约登(Youden)指数最大时即0.756,最佳临界值447.920,敏感度0.903,特异性0.852,见表3。
项目 最佳临界值 敏感度 特异性 约登指数 AUC(95%CI) P值 发病天数(t/d) 14.500 0.387 0.951 0.338 0.722(0.614~0.831) 0.001 淋巴细胞计数 1.685 0.355 0.934 0.289 0.582(0.449~0.715) 0.202 AST 31.950 0.839 0.557 0.396 0.676(0.566~0.786) 0.006 CRP 104.500 0.548 0.869 0.417 0.772(0.672~0.871) <0.001 联合呼吸机辅助通气 0.500 0.806 0.820 0.626 0.813(0.715~0.911) <0.001 联合预测因子 447.920 0.903 0.852 0.756 0.939(0.885~0.993) <0.001 -
Paxlovid是奈玛特韦片150 mg和利托那韦片100 mg组成的组合包装。奈玛特韦是一种SARS-CoV-2主要蛋白酶Mpro3C-样蛋白酶(3CLpro)的拟肽类抑制剂,能抑制病毒复制。利托那韦是HIV-1蛋白酶抑制剂,对新冠病毒SARS-CoV-2 Mpro无活性,但可以通过抑制肝药酶CYP3A介导的奈玛特韦代谢,升高奈玛特韦血药浓度,而发挥协同作用[7]。本研究中,33.70%使用Paxlovid治疗的COVID-19患者出现早期预后不良,其原因可能与药物特点和患者因素有关。据文献报道[8],大量Mpro突变可能诱导对Paxlovid的抗药性,从而导致治疗失败影响患者预后,但另有研究表明,在对731份样本进行分析后,并未发现Mpro突变和Paxlovid治疗失败之间存在显著的联系[9]。同时,有文献报道Paxlovid治疗后7 d和30 d的COVID-19复发率分别为3.53%和5.40%[10],通常患者的病情较轻,但仍有1.35%的患者出现呼吸衰竭需入住ICU[11]。
患者的发病天数是影响Paxlovid早期预后不良的独立危险因素之一。《新型冠状病毒感染诊疗方案(试行第10版)》推荐Paxlovid用于发病5 d以内的轻、中型且伴有进展为重症高风险因素的成年患者[6]。有文献报道发病时间超过5 d,核酸检测CT值<30者也可使用Paxlovid[12]。但在变异毒株奥密克戎感染的情况下,绝大多数二次传播发生在症状出现后5 d之内,在症状出现后8 d未检测到传染性病毒[13-14]。因此,Paxlovid治疗发病5 d后患者的有效性和安全性目前尚不明确。本研究结果显示,患者发病时间越长,Paxlovid治疗的预后越差。
一项针对国内125例COVID-19住院患者临床特征的研究显示,1/3的患者出现淋巴细胞计数降低,约20%患者存在不同程度的ALT和AST升高,70.4%患者的CRP高于正常范围,且与非危重患者相比,危重患者的淋巴细胞水平更低,CRP、IL-6等炎症指标更高,差异具有统计学意义(P<0.05)[15]。从本研究结果同样可以看出,较低的淋巴细胞计数和较高的AST、CRP均提示COVID-19患者进展为危重型的风险高,故使用Paxlovid治疗的预后效果较差。
联合呼吸机辅助通气仅适用于出现呼吸衰竭的危重型COVID-19患者。本研究结果显示,Paxlovid联合呼吸机辅助通气治疗时早期预后不良的风险高,推测可能与重型、危重型COVID-19患者使用Paxlovid抗病毒治疗效果不佳有关。Paxlovid说明书及第10版诊疗方案仅推荐Paxlovid用于有重症高风险因素的轻至中度COVID-19患者。Paxlovid在国外获批上市主要基于一项EPIC-HR研究,该研究纳入的人群同样是重症高风险因素、轻至中度成年的COVID-19患者[9]。因此,对于重型、危重型COVID-19患者使用Paxlovid的循证依据不足。
目前仍缺乏构建预测模型以评估Paxlovid治疗后早期预后不良的风险。根据本研究所构建方程发现,当Y联合预测因子值大于447.920时,提示Paxlovid治疗后早期预后不良的风险高,建议采取更积极的治疗措施包括联合其他抗COVID-19药物等。本研究可能存在不足之处,未来可通过大规模的临床应用和更多临床研究数据提供足够的循证依据。
Risk factors of poor early prognosis in the treatment of COVID-19 with nematevir and ritonavir tablets and the establishment of prediction model
doi: 10.12206/j.issn.2097-2024.202303038
- Received Date: 2023-03-28
- Rev Recd Date: 2023-10-07
- Available Online: 2023-11-25
- Publish Date: 2023-11-25
-
Key words:
- Naimatwe/Litonavir /
- COVID-19 /
- poor prognosis /
- risk factor /
- prediction model
Abstract:
| Citation: | HUANG Wenhui, XU Yanyu, HAO Xiaowei, LIN Guan, OUYANG Shandan, WANG Jiakun, CHEN Jinshan. Risk factors of poor early prognosis in the treatment of COVID-19 with nematevir and ritonavir tablets and the establishment of prediction model[J]. Journal of Pharmaceutical Practice and Service, 2023, 41(11): 700-704. doi: 10.12206/j.issn.2097-2024.202303038 |









 DownLoad:
DownLoad: