-
骨质疏松症和阿尔茨海默病均为退行性疾病,越来越多的研究表明它们的发病机制具有关联[1]。骨质疏松症往往伴随着认知缺陷,Aβ沉积是阿尔茨海默病的典型症状,而阿尔兹海默病小鼠(APPswe/PS1dE9)的骨组织中也会出现Aβ沉积,并出现骨密度降低,骨强度减弱等骨质疏松症状[2]。另有研究表明,氧化应激会导致 Aβ沉积,这种氧化损伤状态可被抗氧化剂改善[3]。因此, Aβ沉积偶联氧化损伤可视为阿尔茨海默病及骨质疏松症的共同发病机制。巴戟天丸收载于明代《古今医统大全》,由君药巴戟天,臣药远志、石菖蒲、茯苓、人参以及地骨皮、茯神组成[4]。前期课题组研究已证实,巴戟天丸组方可以从体内外水平上改善D-半乳糖(D-gal)引发的骨丢失,但巴戟天丸组方治疗Aβ沉积所致的骨丢失及具体作用机制有待进一步阐明[4, 5]。因此,本研究拟以Aβ1-42寡聚体损伤成骨细胞模型,对巴戟天丸组方的抗氧化能力及对骨形成干预作用进行探究,并通过网络药理学方法对潜在的作用机制进行预测。
-
取质量比为5∶10∶10∶10∶10∶10∶3的巴戟天、茯苓、茯神、地骨皮、远志、石菖蒲、人参粉末,混合均匀。按照料液比1∶10加入去离子水,浸泡1 h后,加热煎煮,回流提取2次,每次1 h,所得药液过滤,合并两次的滤液。将收集到的滤液减压浓缩成浓度为2.6 g(生药量)/ml的巴戟天丸水提物母液。-20°C保存备用。
-
新生 24 h Wistar 大鼠(上海西普尔-必凯实验动物有限公司);胎牛血清(以色列BI);MTT(上海碧云天);BCIP/NBT 碱性磷酸酯酶显色试剂盒(上海碧云天)、ALP、CAT、SOD、GSH 、MDA 试剂盒(南京建成);PBS(天津灏洋);DMSO(上海博光);α-MEM 培养基(上海富衡); BMP2、RUNX-2、OPG、GAPDH抗体(美国Abcam)。
-
采用二次消化法从新生 24 h Wistar 大鼠的颅盖骨中分离得到原代成骨细胞,将成骨细胞培养于 α-MEM 培养基中(10% 胎牛血清),置于 37 ℃、5% CO2恒温培养箱中培养,取第 3~5 代成骨细胞进行后续实验。
-
将成骨细胞以 2×104个/孔接种于无菌 96 孔板中,孵育过夜。空白组和模型组更换新的完全培养基,阳性药组加入含N-乙酰半胱氨酸(NAC)的完全培养液(1 mmol/L), 给药组分别加入含不同浓度的巴戟天丸组方完全培养液(0.008、0.04、0.2、1、5 μg/ml),4 h 后模型组和给药组给予Aβ1-42寡聚体进行损伤,使完全培养基中Aβ1-42寡聚体浓度达到 20 mmol/L。培养 48 h 后,采用 MTT 法测定细胞增殖水平。
-
ALP 活性检测:按照“1.4.2”项的方法进行给药,培养 48 h 后,取上清液,根据说明书进行 ALP 活性测定。将成骨细胞以 1×105 个/孔接种于无菌6孔板中,孵育过夜,细胞完全贴壁后,空白组和模型组更换新的完全培养基,阳性药组加入含NAC的完全培养液(1 mmol/L), 给药组分别加入含不同浓度的巴戟天丸组方完全培养基(0.2、1、5 μg/ml),4 h 后模型组和给药组给予Aβ1-42寡聚体进行损伤,使培养基中Aβ1-42寡聚体浓度达到20 mmol/L。培养 48 h 后进行染色,室温下避光孵育 48 h,洗去工作液后置于显微镜下拍照。
-
将成骨细胞以2×104个/孔和5×104 个/孔分别接种于接种于无菌96孔和24孔板中,孵育过夜,细胞完全贴壁后,空白组和模型组更换新的完全培养基,阳性药组加入含NAC的完全培养液(1 mmol/L), 给药组分别加入含不同浓度的巴戟天丸组方完全培养液(0.2、1、5 μg/ml),4 h 后模型组、阳性药组和给药组给予Aβ1-42寡聚体进行损伤,使培养基中Aβ1-42寡聚体浓度达到20 mmol/L。培养 48 h 后根据说明书进行检测。
-
将细胞以 2×105 个/孔接种于 6 孔板中,孵育过夜,细胞完全贴壁后,阳性药组加入含NAC的完全培养液(1 mmol/L), 给药组分别加入含不同浓度的巴戟天丸组方完全培养基(0.2、1、5 μg/ml),4 h 后模型组和给药组加入Aβ1-42寡聚体,使完全培养基中Aβ1-42寡聚体浓度达到20 mmol/L。48 h 后,吸去培养基,PBS 洗涤 3 次。于冰上对细胞进行裂解提取总蛋白,采用 BCA 试剂盒测定蛋白浓度。蛋白变性后,经 SDS-PAGE 凝胶电泳后转移至 PVDF 膜进行转膜,室温封闭1 h后,加入相应的一抗,4 ℃过夜,1×TBST洗涤 3 次,加二抗于室温孵育 50 min,1×TBST洗涤 3 次,采用 ECL 化学发光试剂盒检测。
-
使用 SPSS Staistics 24 统计分析软件进行统计学分析。计量数据均采用(
$\bar{x} $ ±s)表示,选用单因素方差分析(One-Way ANOVA)进行组间变量的比较分析。使用 Graphpad prism 9.0 软件进行统计及绘图。以P<0.05 为差异有显著性意义。 -
通过TCMSP(http://tcmsow.com/tcmsp.php)和ETCM数据库(http://www.tcmip.cn/ETCM/index.php),查找巴戟天、地骨皮、茯苓、人参、石菖蒲和远志6味中药的成分。在TCMSP数据库中,选择药物口服利用度(OB)≥30%,类药性(DL)≥0.18的成分,在ETCM数据库中根据DrugLikeness Grading评分,选择评分为Moderate和Good的成分[6]。
-
通过Uniprot(http://www.uniprot.org/)数据库,将靶点映射成基因,利用Cytoscape 3.6.0软件绘制成分靶点图。
-
通过Disgenet数据库(http://www.disgenet.org/)和Genecards数据库(https://www.genecards.org/)查找阿尔兹海默病和骨质疏松症相关的疾病靶点,绘制PPI蛋白相互作用网络图,整合两大数据库中的靶点基因,去除重复的靶点。利用Venny2.1,将阿尔兹海默病和骨质疏松症相关的疾病靶点图进行交集分析。
-
利用Venny2.1,将阿尔兹海默病、骨质疏松症与巴戟天丸组方相关靶点进行交集分析。将药物疾病交集靶点上传到String数据库进行分析,绘制PPI蛋白相互作用网络图。
-
采用Metascape数据库,对关键靶点进行GO基因富集分析(http://geneontology.org/)和KEGG代谢通路分析(http://www.genome.jp/kegg/),分析巴戟天丸中的主要分子生物过程和信号通路。
-
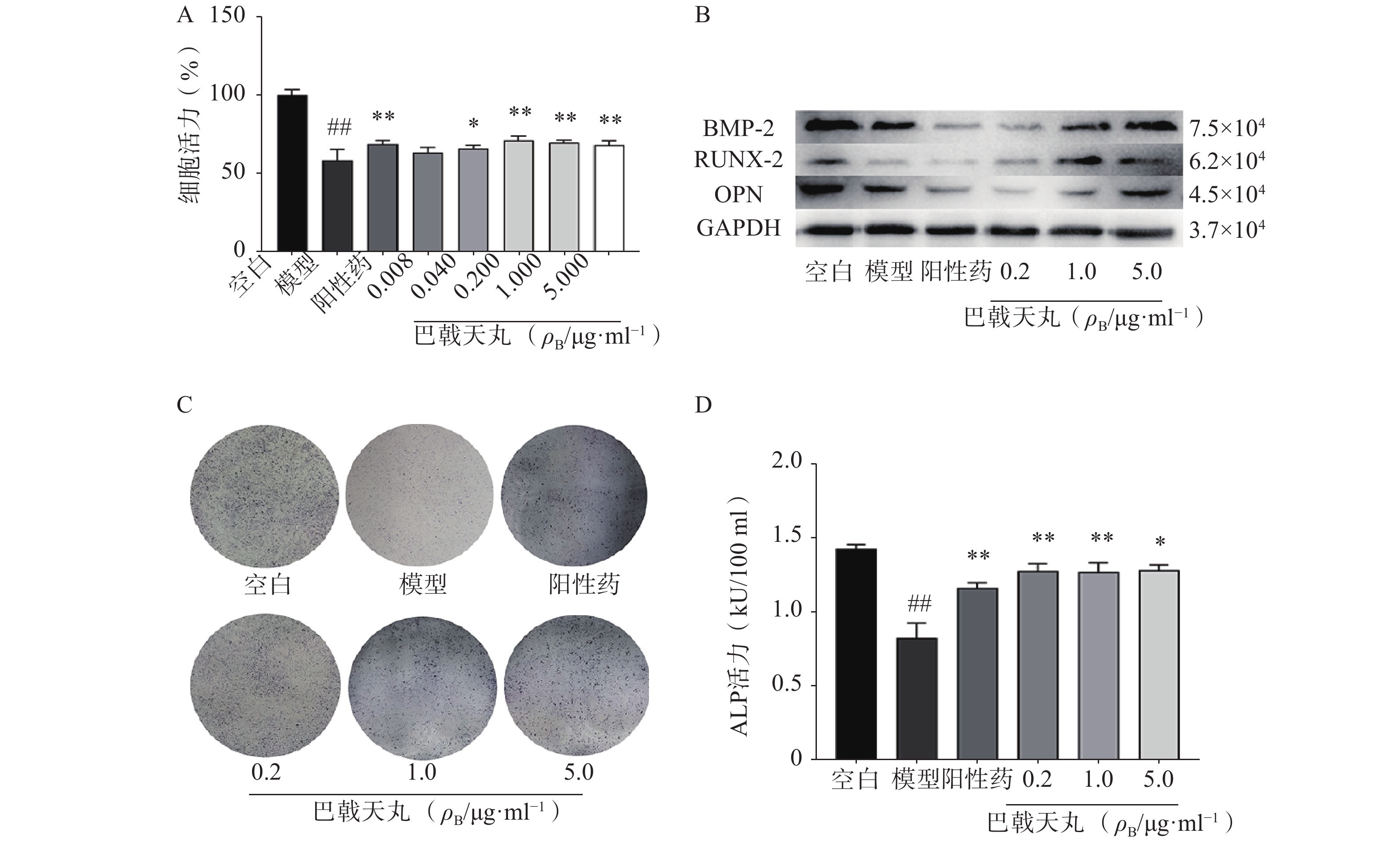
Aβ1-42寡聚体可显著抑制成骨细胞的增殖;巴戟天丸组方在 0.04、0.2、1、5 μg/ml浓度下均能够显著提高成骨细胞的增殖水平(图1A)。Aβ1-42寡聚体会显著抑制成骨细胞中骨形成相关蛋白BMP2、RUNX-2、OPG的表达;给予巴戟天丸组方干预后,BMP2、RUNX-2、OPG的表达显著提高,提示巴戟天丸组方可显著促进Aβ1-42寡聚体损伤成骨细胞的骨形成(图1B)。与空白组相比,Aβ1-42寡聚体可以显著降低成骨细胞的 ALP 活性,而巴戟天丸组方显著逆转了 Aβ 损伤成骨细胞的 ALP 活性,促进成骨细胞的分化(图1C、D)。
-
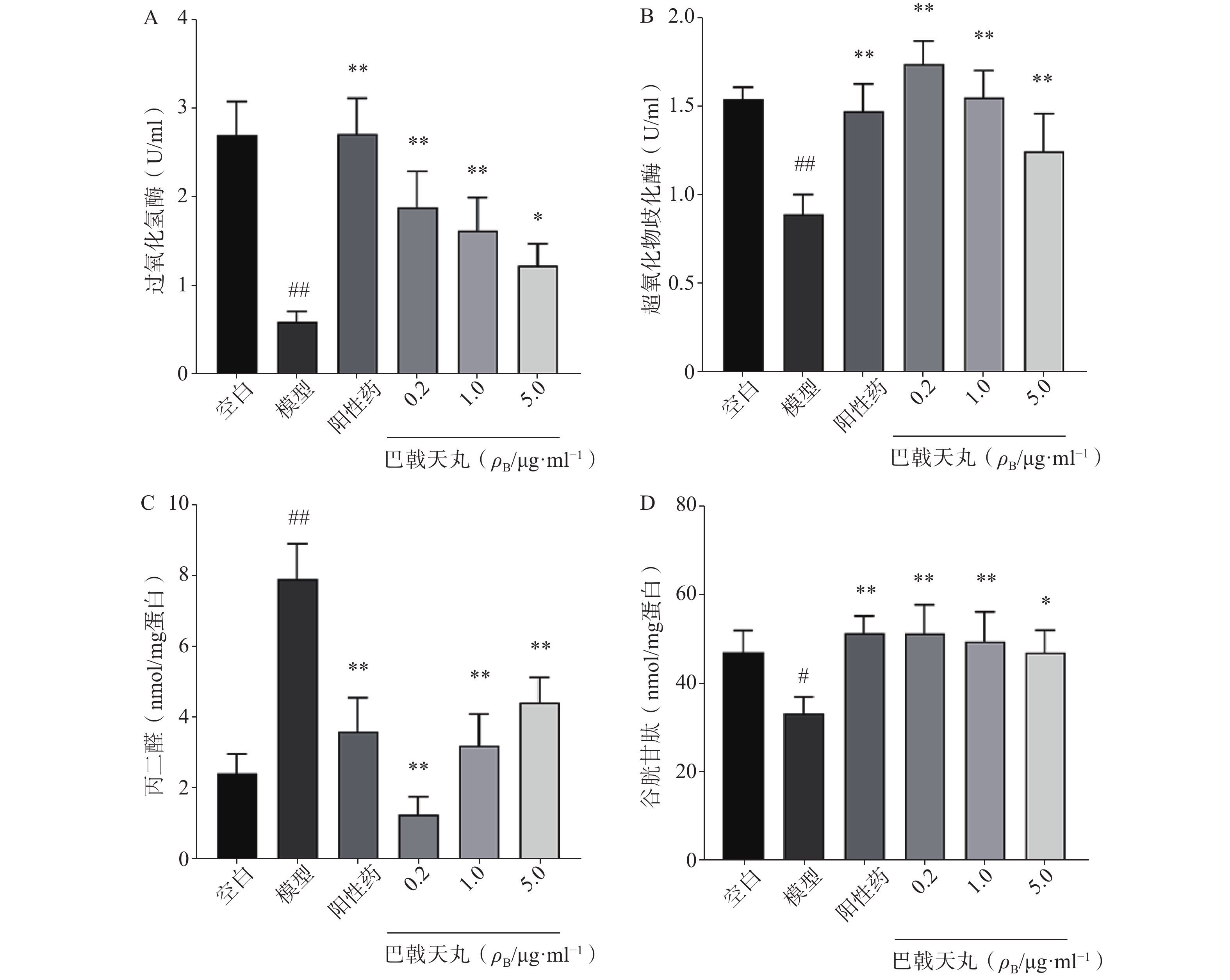
如图2 所示,Aβ1-42寡聚体显著降低了成骨细胞CAT(图2A)、SOD(图2B)、GSH(图2D)的活性,提高MDA的活性(图2C),导致成骨细胞的氧化损伤,而巴戟天丸组方低、中、高剂量均可以显著改善Aβ1-42寡聚体导致的氧化损伤。
-
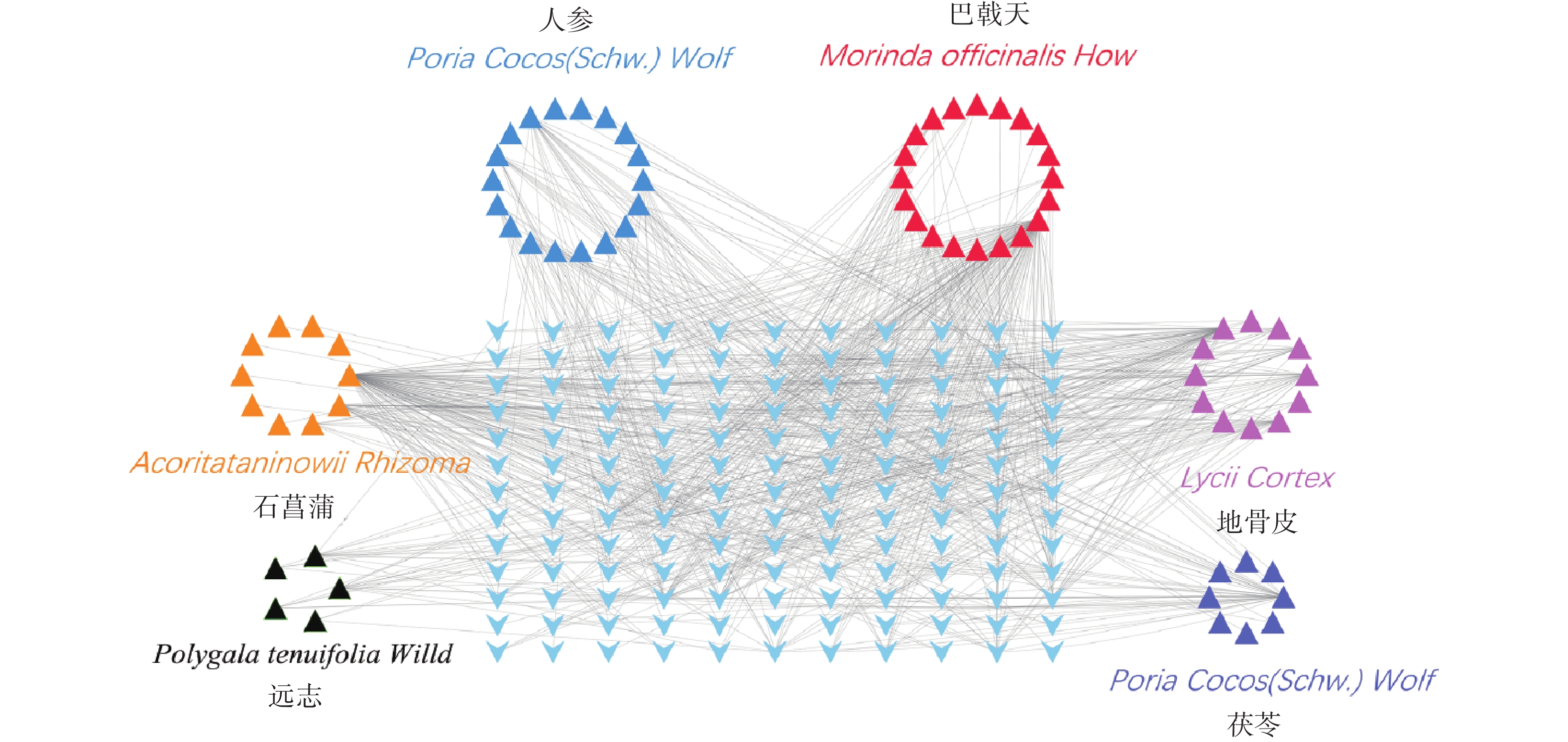
根据条件筛选后,其中,巴戟天成分为20种,地骨皮成分为12种,茯苓成分为10种,人参成分为18种,石菖蒲成分为10种,远志成分为5种,茯神成分0种,共计成分75种。
-
通过TCMSP和ETCM数据库查找满足筛选条件的化学成分对应的靶点,得到151个预测靶点。删除重复靶点后,通过Uniprot数据库映射成基因ID,剔除重复靶点后,得到143个基因symbol(图3)。
-
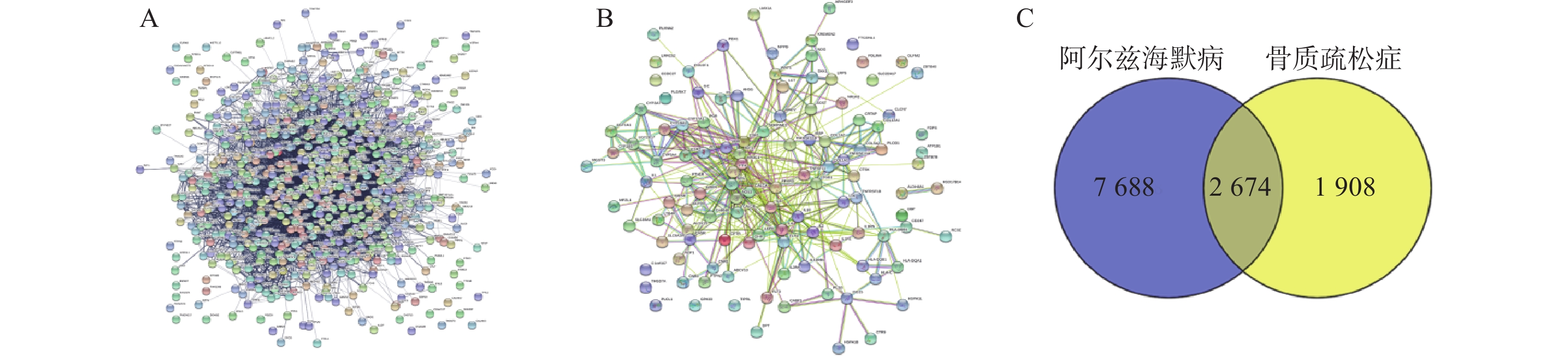
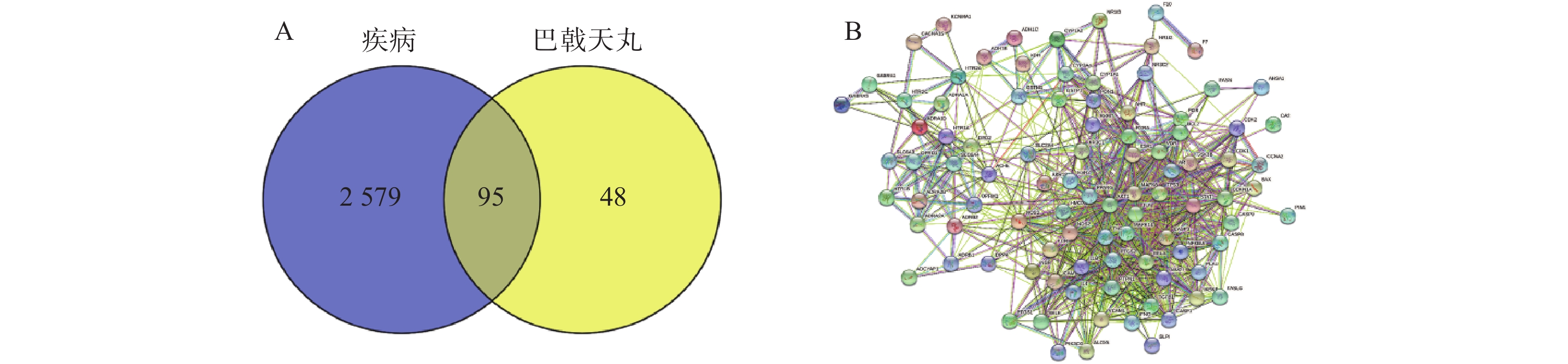
通过Disgenet数据库和Genecards数据库查找得到阿尔兹海默病相关疾病靶点10 362个,骨质疏松症相关疾病靶点4 582个,并将所得靶点进行蛋白相互作用网络分析(图4A、B)。将阿尔兹海默病和骨质疏松症相关的疾病靶点图进行交集分析,得到共有靶点2 674个(图4C)。
-
将2 674个疾病交集靶点基因和143个药物成分靶点基因再次进行交集分析,得到共有靶点95个(图5A),将95个药物疾病交集靶点上传到String数据库进行分析,绘制PPI蛋白相互作用网络图。将单独节点去除后,图中共有94个节点,871条边,意味着它们间有特殊的关联,即蛋白质共同贡献一个共同的功能,每个靶点的平均节点度为18.3,说明交集靶点间关系密切而且存在很强的相互作用关系(图5B)。
-
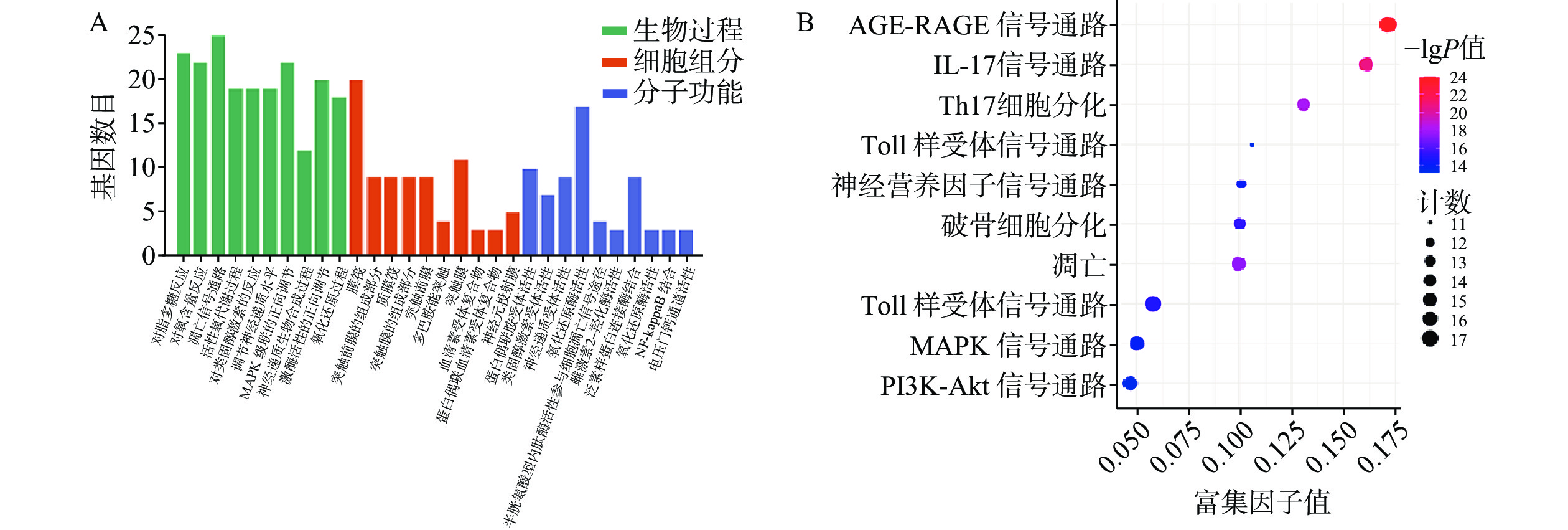
GO富集分析包括生物过程(BP)、分子功能(MF)及细胞组分(CC),将每个结果前 10 的条目可视化为条形图(图6A)。巴戟天丸活性物质参与的BP主要包括对脂多糖的反应、对氧含量的反应、细胞凋亡信号途径、活性氧代谢过程。MF主要包括G蛋白偶合胺受体的活性、神经递质受体的活性、氧化还原酶活性、参与凋亡信号通路的半胱氨酸型内肽酶活性、泛素类蛋白连接酶结合。CC主要包括膜筏、突触膜的整体组成部分、浆膜筏等。 KEGG 通路富集分析中主要涉及与阿尔兹海默症、骨质疏松症相关的通路有AGE-RAGE信号通路、PI3K-Akt信号通路、MAPK信号通路以及神经活性配体与受体的相互作用通路等(图6B)。
-
骨骼重塑是一个重要的生理过程,其主要包括两个阶段,成骨细胞主导的骨形成和破骨细胞主导的骨吸收[7]。其中,成骨细胞的增殖能力反映骨形成的强弱,其分泌的 ALP 是分化阶段的关键酶,可促进骨组织矿化[8]。本研究中,各剂量巴戟天丸均可显著改善Aβ损伤成骨细胞的增殖抑制,且0.2 μg/ml效果最好,5 μg/ml的效果优于0.04 μg/ml,故选择0.2 μg/ml、1 μg/ml、5 μg/ml作为巴戟天丸给药剂量进行后续实验,并发现其可显著提高Aβ损伤成骨细胞的ALP活性和骨形成相关蛋白 BMP2、RUNX-2、OPG的表达,促进骨形成。GSH是细胞抗氧化系统的一个重要成员,高水平的GSH对于清除过多的活性氧(ROS)和解毒异物是不可缺少的[9]。MDA是细胞中多不饱和脂肪酸过氧化的最终产物之一,自由基的增加会导致MDA的过度生产,加剧氧化损伤[10]。SOD和CAT是重要的酶类抗氧化剂,二者活性的降低会导致氧化损伤[11,12]。本实验中巴戟天丸组方可逆转Aβ损伤成骨细胞GSH、SOD、CAT活性的降低, MDA活性的升高,结果所表现的剂量依赖与增殖实验相吻合,表明巴戟天丸组方可作为抗氧化剂改善成骨细胞的氧化损伤。后采用网络药理学方法进一步探究巴戟天丸干预Aβ损伤成骨细胞的作用机制,结果显示,巴戟天丸促进Aβ损伤状态下成骨细胞骨形成的作用可能与AGE-RAGE、PI3K-Akt及MAPK等信号通路有关。其中,AGE-RAGE信号通路的激活会扰乱细胞的氧化还原平衡并调节各种细胞死亡途径 [13]。作为AGE-RAGE信号通路的下游, PI3K-Akt信号通路的激活会增强细胞的抗氧化能力,保护细胞免受氧化应激[14]。此外,MAPK通路的激活同样可以减轻氧化应激状态[15],故网络药理学研究结果同样提示巴戟天丸组方可通过缓解氧化损伤发挥干预Aβ沉积损伤成骨细胞的作用。
The roles of Bajitianwan formula on Aβ-injured osteoblasts and the mechanism based on network pharmacology
doi: 10.12206/j.issn.2097-2024.202305011
- Received Date: 2023-05-08
- Rev Recd Date: 2024-01-26
- Available Online: 2024-07-16
- Publish Date: 2024-07-25
-
Key words:
- osteoblasts /
- Bajitianwan /
- Aβ deposition /
- network pharmacology
Abstract:
| Citation: | JIANG Tao, XU Weifan, JIANG Yiping, XIA Tianshuang, XIN Hailiang. The roles of Bajitianwan formula on Aβ-injured osteoblasts and the mechanism based on network pharmacology[J]. Journal of Pharmaceutical Practice and Service, 2024, 42(7): 285-290, 296. doi: 10.12206/j.issn.2097-2024.202305011 |









 DownLoad:
DownLoad: