-
盐酸阿霉素是一种基于蒽环类的广谱抗肿瘤药物,能够通过多种机制达到抗肿瘤活性。他能与S期DNA相互作用,抑制核酸合成。此外,作为DNA拓扑异构酶Ⅱ抑制剂,盐酸阿霉素可引起DNA链的断裂[1-5];但由于盐酸阿霉素严重的心脏毒性和肿瘤细胞耐药性阻碍了其在临床实践中的广泛应用。小分子化疗增敏剂能够改善肿瘤的多药耐药,提高化疗药物的疗效。氯尼达明在调节热疗、放射疗法、光动力疗法的活性方面具有潜力[6-7]。氯尼达明作为己糖激酶Ⅱ(HK-Ⅱ)抑制剂,能够抑制肿瘤细胞有氧糖酵解、减少肿瘤细胞的能量供应[8-9]。此外,氯尼达明可以通过破坏跨膜电位而有效地触发线粒体凋亡途径。但它作为单一药物,其抗肿瘤活性有限[10],因此经常与其他抗肿瘤药物联合用作化学增敏剂。有研究报道指出,氯尼达明与盐酸阿霉素的共递送,在体外能够显著增强细胞毒性并降低抗肿瘤药物的给药剂量[11-14],二者在协同治疗肝癌、卵巢癌、乳腺癌等领域已取得显著进展。通过大量的文献调研,我们发现氯尼达明与盐酸阿霉素的最佳协同比例为1∶6,但并未有人专门构建氯尼达明与盐酸阿霉素混合溶液的含量分析方法[13]。因此,本研究采用HPLC法建立针对氯尼达明与盐酸阿霉素联合用药的含量分析测定方法,为二者联合用药的含量检测提供参考。
-
Agilent-1260 Infinity Ⅱ高效液相色谱仪、紫外可见分光光度计[安捷伦科技(中国)有限公司];SECURA125-1CN型十万分之一电子天平、Arium@mini超纯水机(德国赛多利斯)。
-
盐酸阿霉素对照品(99%)、甲醇(色谱纯)、三氟乙酸(TFA,色谱纯)均购自sigma-Aldrich Company;氯尼达明对照品(99%,上海毕得医药科技有限公司);纯净水(杭州娃哈哈集团有限公司)。
-
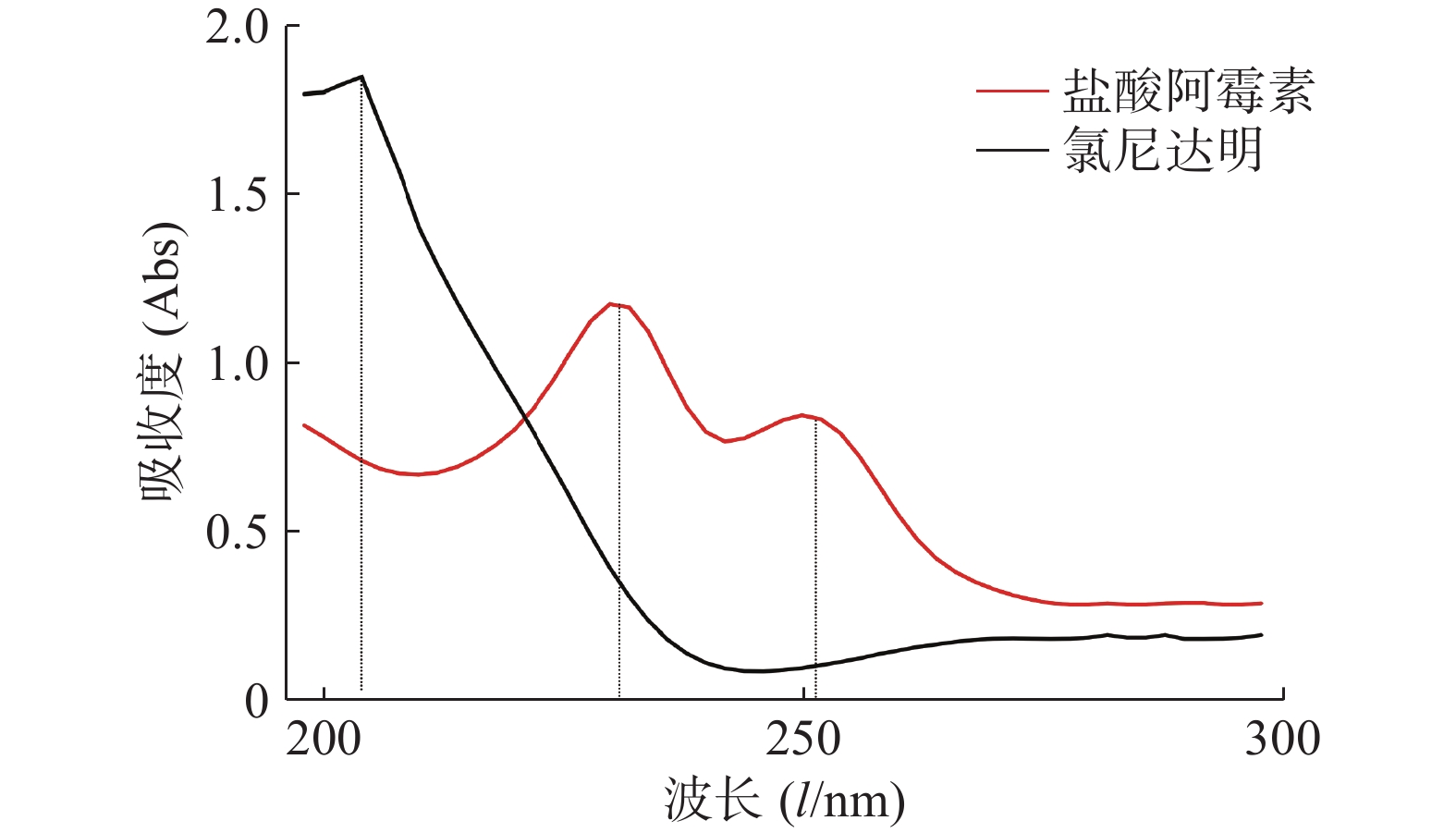
精密称取氯尼达明和盐酸阿霉素对照品适量,分别用甲醇水溶液(50%)溶解制成20 µg/ml的对照品溶液,在190~400 nm波长内进行扫描,盐酸阿霉素的最大吸收波长为228、253 nm,氯尼达明的最大吸收波长为205 nm。故分别选择253、205 nm作为检测波长,见图1。
-
色谱柱:Agilent 5 HC-C18(2)(4.6 mm×250 mm,5 µm);流动相:甲醇(A)-0.1% TFA水溶液(B);检测波长:205、253 nm;流速: 1.0 ml/min;柱温:35℃;进样量:10 µl;梯度洗脱程序如表1所示。
时间(t/ min) 流动相A(%) 流动相B(%) 0 65 35 3 65 35 7 90 10 13 90 10 15 65 35 20 65 35 -
精密称取12 mg 氯尼达明加入50%甲醇水溶液,涡旋震荡并超声溶解后定容至10 ml量瓶中,即得1.2 mg/ml氯尼达明溶液;精密称取10 mg盐酸阿霉素加入50%甲醇水溶液,涡旋震荡溶解后定容至50 ml量瓶中,即得0.2 mg/ml盐酸阿霉素溶液。
-
将“2.3.1”项中的溶液等体积混合后,量取一定体积的50%甲醇水溶液稀释2.5倍,定容于10 ml容量瓶中,即得氯尼达明与盐酸阿霉素质量浓度分别为240 µg/ml和40 µg/ml的混合溶液。
-
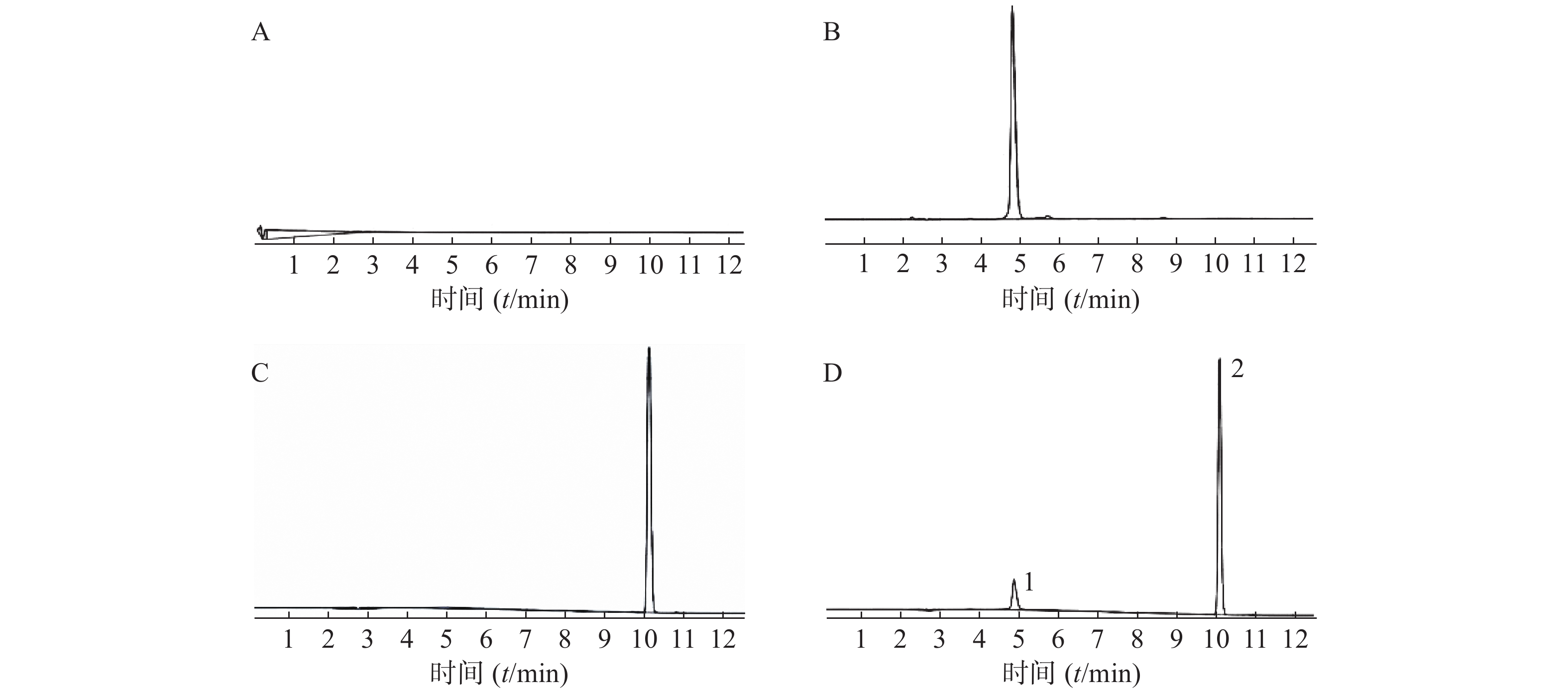
按照“2.3.1”项及“2.3.2”项条件,配制以下样品。按照“2.2”项下方法进样检测,考察方法的专属性。如图2所示,实验结果表明,该方法专属性良好,氯尼达明与盐酸阿霉素的色谱峰完全分离。
-
精密量取不同体积“2.3.2”项下制备的混合对照品溶液,置于5 ml量瓶中,加溶剂稀释并定容,最终得到质量浓度为240、180、120、60、30、12、6 µg/ml的氯尼达明溶液,此条件下,盐酸阿霉素的质量浓度分别为40、30、20、10、5、2、1 µg/ml。
按上述“2.2”项下色谱条件进样,以对照品的峰面积(Y)对质量浓度(X)进行线性回归,得氯尼达明回归方程: Y=51.439X+111.46,r=0.9999,证明在本方法下,氯尼达明在6~240 µg/ml 浓度范围内线性良好。得盐酸阿霉素回归方程:Y= 25.142X + 2.1863,r=0.9999,证明在本方法下,盐酸阿霉素在1~40 µg/ml浓度范围内线性良好。
-
取氯尼达明质量浓度为30、60、240 µg/ml的混合对照品溶液,按照“2.2”项下的色谱条件进行日内精密度及日间精密度考察。日内精密度测定方法为样品测定5次,计算日内相对偏差;日间精密度测定方法为样品连续测定5 d,计算日间相对偏差。如表2、表3所示,3种不同浓度的氯尼达明和盐酸阿霉素的日内、日间精密度的RSD值均小于2.0 %,符合精密度要求。
浓度(ρB/µg·ml−1) 日内RSD(%) 日间RSD(%) 5 1.40 1.48 10 0.38 0.92 40 0.11 0.06 浓度(ρB/µg·ml−1) 日内RSD(%) 日间RSD(%) 30 0.03 0.19 60 0.13 0.06 240 0.11 0.07 -
根据上述方法配制质量浓度分别为60、10 µg/ml 的氯尼达明、盐酸阿霉素混合溶液,分别在第0、2、4、8、12、24 h时进样,按照“2.2”项下色谱条件,进行含量测定,结果如表4、表5所示,表明氯尼达明与盐酸阿霉素的混合溶液在8 h内稳定。
时间(t/h) RSD(%) 0 0.20 2 0.07 4 0.05 8 0.13 12 0.12 24 0.02 时间(t/h) RSD(%) 0 0.32 2 0.38 4 0.14 8 0.49 12 0.62 24 0.58 -
精密称取6份12 mg 氯尼达明和10 mg 盐酸阿霉素,加入50%甲醇水溶液,分别定容于10 ml和50 ml容量瓶中,即得1.2 mg/ml 氯尼达明溶液和0.2 mg/ml 盐酸阿霉素溶液。
将上述溶液等体积混合后,量取一定体积的50%甲醇水溶液稀释2.5倍,定容于10 ml容量瓶后,即得6份氯尼达明与盐酸阿霉素质量浓度分别为240 µg/ml和40 µg/ml的混合溶液,按“2.2”项下色谱条件进行测定,带入回归方程计算氯尼达明和盐酸阿霉素的含量、回收率和RSD值。结果如表6所示。说明此方法回收率符合要求。
成分 原有量
(m/mg)测得量
(m/mg)回收率
(%)平均回收率
(%)RSD
(%)氯尼达明 12.0 11.8 98.3 99.2 2.9 12.1 11.9 98.3 11.9 11.8 99.2 12.4 12.8 98.4 11.8 11.8 100.0 12.1 12.8 105.8 盐酸阿霉素 10.1 9.8 97.0 100.8 1.9 10.1 9.8 97.0 10.0 9.8 98.0 9.9 10.0 101.0 10.1 10.0 99.0 10.1 10.2 101.0 -
氯尼达明极性小,有机相比例的改变对其出峰时间影响较大。对于流动相的选择,本实验曾尝试以乙腈(0.1% TFA)-水(0.1%TFA)、乙腈:水(0.1% 三乙胺,磷酸调pH=3.0)、乙腈:水(0.1%甲酸)作为流动相[15-17],但氯尼达明出峰时间过长且峰对称性差。以甲醇-水(0.1% TFA)作为流动相时,分离效果及峰形较好[18],故分别考察了甲醇与水分别为90∶10、80∶20、70∶30、65∶35等比例下两种化合物的保留时间。结果表明,有机相甲醇比例低于70% 时,氯尼达明出峰时间过长。在溶剂的选择上,本实验尝试以甲醇、甲醇水溶液作为溶剂,当溶剂为甲醇水溶液时,盐酸阿霉素及氯尼达明两组不存在前沿及拖尾现象,且基线较平稳,分离度较好。故在此基础上,为缩短氯尼达明的出峰时间,我们尝试在4~15 min内改变有机相比例,利用梯度洗脱以保证两种化合物出峰完整且保留时间适中。本实验结果表明,选用该梯度洗脱方法同时测定氯尼达明和盐酸阿霉素,在一定浓度范围内线性良好,专属性高,且精密度、回收率、稳定性均符合方法学要求,可以作为同时测定氯尼达明和盐酸阿霉素含量的测定方法。
Content measurement of doxorubicin hydrochloride and lonidamine by HPLC
doi: 10.12206/j.issn.2097-2024.202306043
- Received Date: 2023-06-27
- Rev Recd Date: 2023-12-07
- Available Online: 2024-03-20
- Publish Date: 2024-03-25
-
Key words:
- lonidamine /
- doxorubicin hydrochloride /
- HPLC /
- drug combination /
- gradient elution
Abstract:
| Citation: | SUN Yuhan, XU Ziyi, LIAO Jun, ZHANG He, FAN Li, LU Ying. Content measurement of doxorubicin hydrochloride and lonidamine by HPLC[J]. Journal of Pharmaceutical Practice and Service, 2024, 42(3): 127-130. doi: 10.12206/j.issn.2097-2024.202306043 |








 DownLoad:
DownLoad: