-
全球癌症统计数据表明,肝癌是全球第二大癌症死亡原因,因肝癌死亡的人口数占癌症总死亡人数的8.2%。肝细胞癌是原发性肝癌最常见的形式[1],手术是其首选的治疗方法,然而只有5%~10%的肝细胞肿瘤适合切除,大多数患者(50%~70%)在手术后5年内发生肿瘤复发[2]。在小分子药物治疗方面,多激酶抑制剂索拉非尼能够延长患者近3个月中位生存期和进展时间[3],但仍无法满足临床治愈肝细胞癌的需求。因此,寻找新的肝细胞癌治疗靶点,开发新的治疗策略具有重要的临床意义。
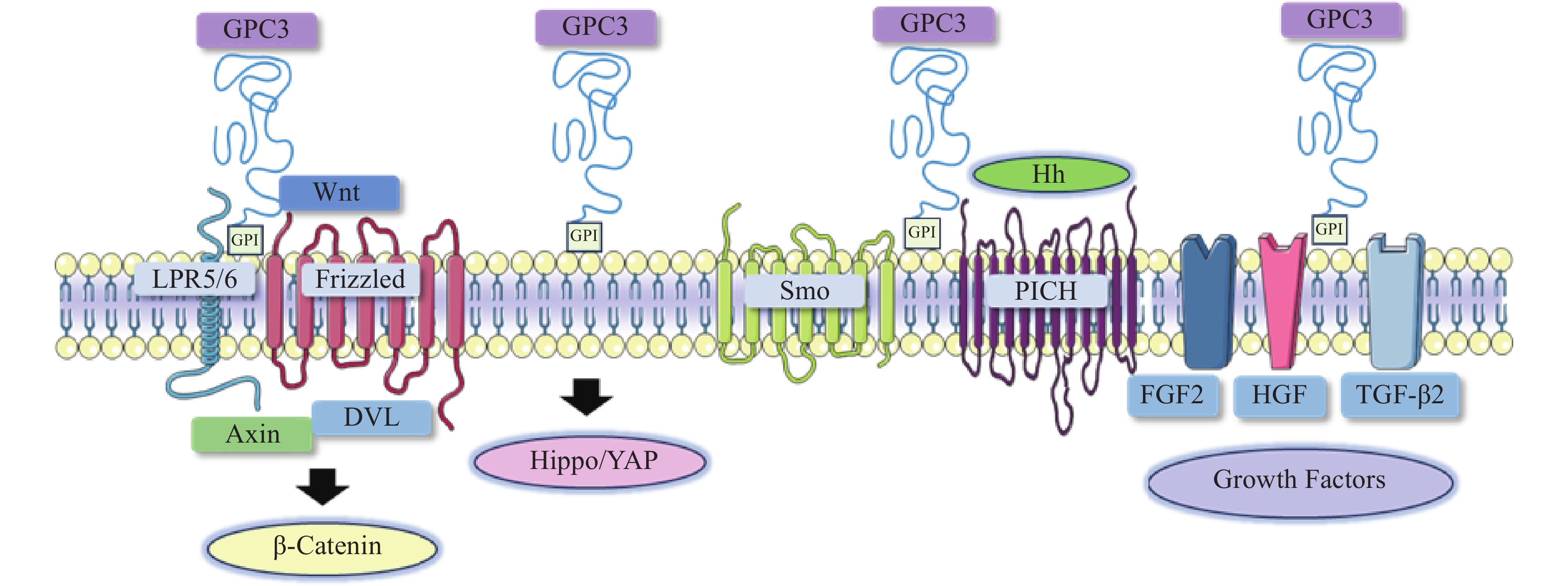
磷脂酰肌醇蛋白聚糖(GPCs),属于硫酸乙酰肝素蛋白多糖家族,通过糖基磷脂酰肌醇(GPI)锚定于细胞膜外,是细胞外基质的主要组成部分[4]。磷脂酰肌醇蛋白聚糖由一个核心蛋白以及与其相连的两条硫酸乙酰肝素(HS)链组成,该家族包括6个成员(GPC1-6)[5]。其中GPC3研究最为广泛,其由580个氨基酸组成,表达于胎儿的肝、肺、胎盘和肾器官,由于DNA甲基化诱导的表观遗传沉默,其在成人器官中的表达明显降低[6]。在临床研究中,GPC3与肿瘤的发生发展密切相关,许多研究都报道了GPC3在肝细胞癌中表达上调[7]。本文综述了GPC3在肝细胞癌相关信号通路中的作用以及基于GPC3开发的肝癌靶向疗法。
-
在肝细胞癌的发生与发展中,经典Wnt信号通路激活是常见的分子事件,与肿瘤细胞存活、增殖、迁移和侵袭密切相关[8]。经典的Wnt信号通路是一个依赖于β-catenin的信号转导过程,由Wnt与两种辅助受体结合而激活,分别是卷曲蛋白(FZD)以及低密度脂蛋白受体相关蛋白5/6(LRP5/6)[9-10]。Wnt结合到FZD富含半胱氨酸的Wnt结合结构域,诱导了FZD与LRP5/6复合物的组装[11]。FZD和LRP5/6构象的改变,以及后续糖原合成酶激酶3、酪蛋白激酶1的磷酸化,促进了破坏复合物重要成分轴抑制蛋白(Axin)的募集。当蓬乱蛋白(DVL)被募集并结合到FZD蛋白的C端尾部时,由DVL、Axin和其他结合辅助因子组成的破坏复合物是稳定的[12-13]。Axin可以防止β-catenin降解,因此,β-catenin在细胞质中积累,进入细胞核,驱动细胞增殖和存活基因的转录[14]。由于Wnt信号通路对肝胆功能、细胞分化和修复等许多功能至关重要,其失调可导致肝细胞癌、肝母细胞瘤或其他肝脏疾病。
在肝细胞癌中,过表达的GPC3作为Wnt蛋白的辅助受体或储存位点来调节细胞表面信号传导(图1)[15]。GPC3可以潜在地激活Wnt信号通路,增加细胞膜上Wnt蛋白数量,或与Wnt及其受体FZD结合,稳定其相互作用,促进复合物的形成。当包含GPC3、Wnt和Frizzled在内的复合物被内吞后,可激活下游信号通路。在上述信号传递过程中,GPC3通过核心蛋白或HS侧链,以依赖于FZD表达水平的方式促进Wnt的激活[16]。此外,GPC3从细胞表面脱落也会影响Wnt信号通路,其调控机制尚不明确,仍需进一步研究。
-
Hippo信号通路在调节细胞大小、器官体积、组织再生和癌症发展中起关键作用[17]。在哺乳动物中,Hippo信号通路涉及两个主要的激酶Mst1/2和Lats1/2。Lats1/2可诱导yes相关蛋白(YAP)磷酸化并使其失活,从而导致cyclin E、diap1和bantam等一系列目标基因下调(图1)。YAP已被证明是经典Hippo信号通路中关键的下游效应分子[18],因其在肝细胞癌中过表达,也被确定为一种致癌基因,可引发并促进HCC的发展。多项研究表明GPC3可能是YAP的靶基因之一,LI等[19]观察到YAP激活程度与肝细胞癌组织GPC3表达呈正相关。此外,研究人员还证明了GPC3敲除在mRNA和蛋白水平上抑制YAP表达,诱导Huh7细胞凋亡,证明GPC3与YAP存在互相调节的关系[20]。
-
Hedgehog(Hh)信号通路在胚胎发育中起着决定性作用,并且在物种间高度保守。Hh信号通路与细胞的生长、分化,以及组织和血管的形成密切相关。经典的Hh信号通路涉及三种Hh配体的募集,分别是:SonicHedgehog(SHH)、Indian Hedgehog(IHH)和Desert Hedgehog(DHH)。Hh信号传递受Patched(PTCH)和Smoothened(SMO)的控制。在正常情况下,PTCH通过抑制SMO蛋白活性,从而抑制下游通路。当PTCH与Hh结合以后,解除对SMO的抑制作用,介导下游信号传导(图1)[21-22]。
当Hh信号通路失调时,可导致肿瘤生长、恶性转化或转移[23-24]。研究人员发现GPC3能以高亲和力结合Shh和Ihh,并与PTCH竞争结合Hh,导致PTCH失活、SMO被持续性抑制,从而阻止Hh信号传导[25]。GPC3可通过抑制Hh信号通路促进HepG2细胞增殖[26]。后续研究还表明,转化酶的切割、LRP1对GPC3抑制Hh信号传导发挥重要的作用[25-26]。
-
GPC3可作为一种辅助受体,通过其HS侧链调节生长因子的活性,刺激细胞生长[27]。AKUTSU等[28]报道了利用小干扰RNA敲低GPC3,导致肝癌细胞中一系列生长因子mRNA和蛋白水平下调。FGFs是一个能广泛影响细胞活动的广谱生长因子家族[29]。在肝癌细胞中,GPC3通过HS侧链,作为FGF2的辅助受体与其结合[30]。LAI等[31]报道了一种肝素降解内酯酶sulfatase 2,能够在肝癌细胞中上调GPC3的表达,并促进FGF信号传导。遗传学研究表明,肝细胞生长因子(HGF)/c-Met通路可调节肝细胞癌的进展(图1)[32]。GAO等[32]证实GPC3通过HS侧链介导的HGF/c-Met通路可影响肝细胞癌的迁移和运动。TGF-β激活素、BMP等转化生长因子(TGF)超家族成员,可调节细胞增殖、细胞凋亡等多种细胞反应。TGF-β与其受体结合后可介导TGF-β信号传导,进而激活下游SMADs及相关复合物。SUN等[33]发现在肝癌细胞中抑制GPC3表达会导致TGF-β2表达增强,从而抑制细胞增殖,阻滞细胞周期进程,诱导复制性衰老。
GPC3在肝癌相关信号通路中发挥着广泛且重要的作用,因此,进一步阐明GPC3在这些信号通路中所发挥的作用、肝癌相关信号通路是如何通过GPC3产生密切联系以调控基本细胞过程等问题,对后续开发靶向治疗策略有着重要意义。
-
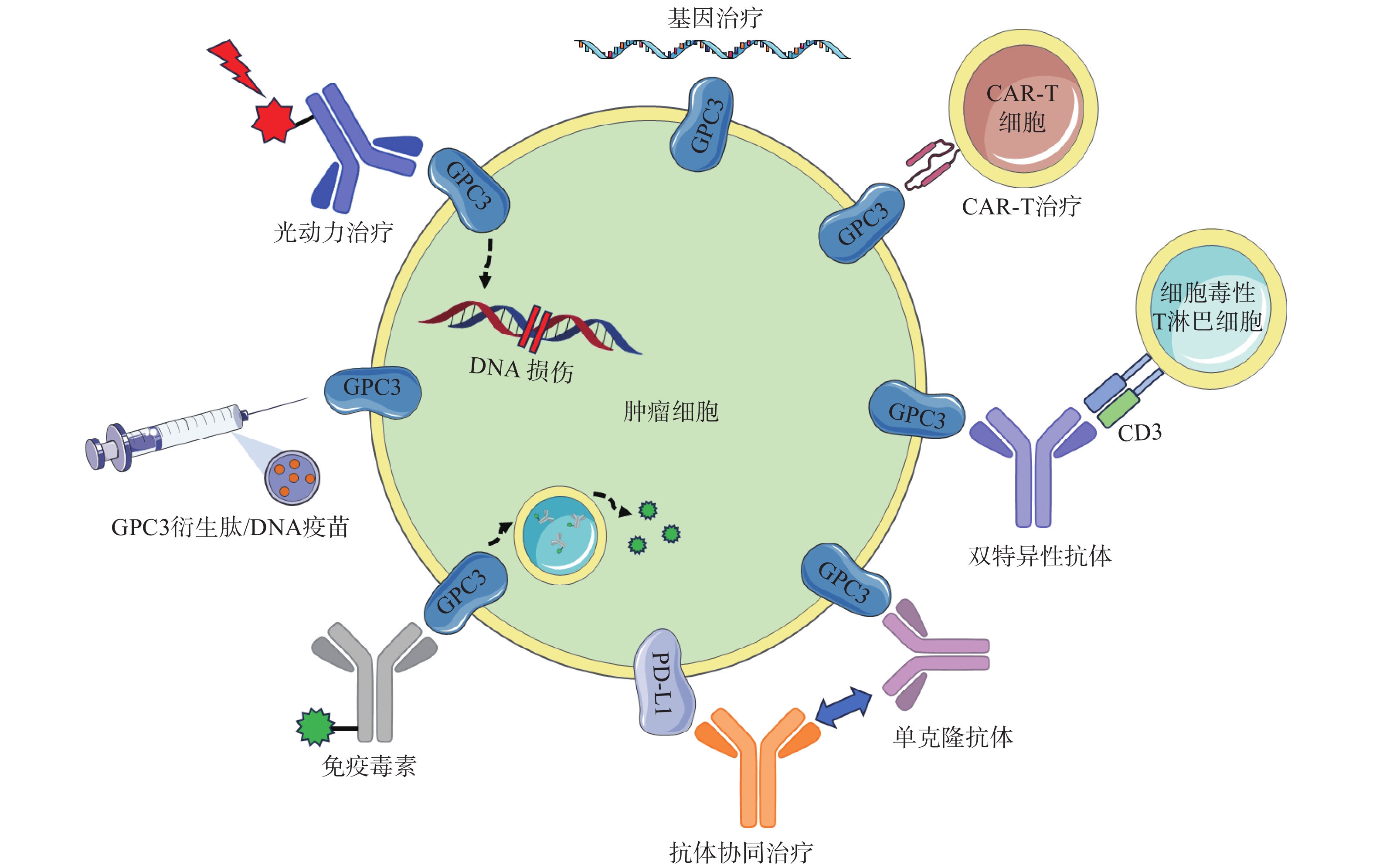
GC33(Codrituzumab)是一种重组人源化单克隆抗体,以高亲和力结合GPC3的近膜结构域(图2)。GC33可诱导产生抗体依赖性细胞毒性(ADCC)、补体依赖性细胞毒性(CDC)以抑制肿瘤生长。GC33能抑制小鼠异种移植瘤生长,其抑制程度与细胞表面抗原水平近似相关[34]。在临床研究中,GC33治疗晚期肝细胞患者的I期剂量递增研究,已分别在美国和日本进行。两项研究都表明,GC33耐受性良好,在最高计划剂量下未检测到剂量限制性毒性[35-36]。然而,在121例肝细胞癌患者的随机II期临床试验中,GC33的治疗并没有获得显著的临床收益。使用GC33与安慰剂相比,中位无进展生存时间分别为2.6个月和1.5个月,中位总生存期分别为8.7个月和10个月[37]。尽管应用更大剂量的GC33,或选择GPC3高表达的患者进行治疗可能会改善治疗结果,但这些假设还需要进一步的临床研究来验证。
针对GPC3这一靶点,研究人员还开展了其他抗体相关研究工作。LIU等[38]报道了一种特异性靶向GPC3中间结构域的单克隆抗体32A9,可抑制小鼠肝细胞癌的生长。FENG等[39]报道了一种抗体HN3,能以高亲和力识别完整的GPC3蛋白,该抗体能够使细胞周期阻滞于G1期,抑制GPC3阳性细胞的增殖,抑制小鼠异种移植瘤的生长。GAO等[40]开发了一种人源抗GPC3单克隆抗体HS20,可识别GPC3蛋白中的HS侧链,通过阻断Wnt信号传导抑制肿瘤生长,该抗体对小鼠未显示出毒性,安全性较好。尽管GPC3是一种特异性良好的肝癌相关抗原,上述靶向GPC3的抗体在临床前研究工作中展现出了一定的疗效,但仍需开展进一步的临床研究以考察这些抗体的治疗作用与安全性。
-
鉴于靶向GPC3的单克隆抗体有限的临床治疗作用,以及新型免疫治疗药物双特异性抗体的兴起,研究人员针对GPC3开发了双特异性抗体ERY974,一种人源化IgG结构的T细胞重定向抗体(TRAB),具有两条不同的重链和一条共同的轻链,重链可分别识别GPC3和CD3(图2)[41]。ERY974以GPC3依赖的方式激活T细胞,对包括肝细胞癌在内的多种表达GPC3的实体瘤表现出抗肿瘤作用,并对免疫检查点抑制剂治疗无效的肿瘤,也显示出显著的非免疫原性抗肿瘤作用[42]。进一步的研究表明,在异种移植瘤模型中,ERY974和化疗药物的联合使用显著增强了抗肿瘤作用。该双特异性抗体正在进行一项针对GPC3阳性实体瘤患者的临床I期试验(NCT02748837)。DU等[43]报道了一种靶向GPC3和CD47的双特异性抗体,通过识别两种抗原,有效阻止肿瘤生长。与抗CD47单抗相比,双特异性抗体具有较长的血清半衰期,无全身不良反应,且疗效更优。这些结果提示,针对GPC3开发双特异性抗体可能是未来肝癌治疗的热门研究方向。
-
除了开发靶向GPC3的抗体外,研究GPC3衍生肽/DNA疫苗也是一种针对肝癌有价值的治疗策略(图2)。NAKATSURA等[44-45]在肝细胞癌患者中,发现了人类白细胞抗原HLA-A24限制性细胞毒性T淋巴细胞(CTL)表位GPC3298-306肽(EYILSLEEL)以及HLA-A2限制性表位GPC3144-152肽(FVGEFFTDV)。在一项针对晚期肝细胞癌患者的I期临床试验中,GPC3疫苗表现出良好的耐受性,疫苗诱导了高比例的GPC3特异性CTL反应。在33例患者中,1例患者表现出部分缓解(PR),19例疾病稳定(SD)患者中有4例出现肿瘤消退,并超出PR标准。持续治疗2个月后,疾病控制率(PR+SD)为60.6%[46]。一项II期、非盲、单臂试验(UMIN-CTR: 000002614)招募了40名接受手术或射频消融的肝癌患者。在治愈后的1年中,进行了10次疫苗接种,结果表明,接受手术/射频消融与疫苗治疗的患者,肿瘤复发率明显低于仅接受手术治疗的患者(1年复发率:28.6% vs 54.3%,P=0.346)[47]。研究人员还鉴定了5种GPC3衍生的长肽,它们可以诱导HLA II型限制性th1细胞的应答,这些经肽诱导的th1细胞的响应与总生存期的延长密切相关[48]。
除了这些肽疫苗外,GPC3 DNA疫苗也可诱导机体产生针对肝癌细胞的细胞毒性T淋巴细胞,抑制同质性肿瘤生长,并提高异种移植瘤小鼠的存活率[49]。然而,尽管多肽疫苗具有潜在的吸引力,但其对于晚期肝癌的治疗作用太弱。瘤内注射多肽疫苗或将其与抗PD-1抗体联用,可通过增加疫苗诱导的CTL免疫应答来增强GPC3衍生肽疫苗的抗肿瘤效果[50]。
-
免疫毒素是抗体片段与毒素片段融合产生的嵌合结构。假单胞菌外毒素(PE)是一种常见的用于免疫毒素的毒素片段,PE从嵌合分子分离后可诱导蛋白质合成暂停,最终导致细胞死亡。因此,免疫毒素被认为能通过两种机制触发肿瘤消退:抗体诱导的细胞信号失活,以及毒素诱导的蛋白质合成抑制(图2)[51]。GAO等[52]构建了免疫毒素HN3-PE38和YP7-PE38,后者通过抑制Wnt/β-catenin信号通路以及YAP信号通路,在HepG2细胞中发挥有效的抗肿瘤作用。然而,免疫毒素潜在的脱靶效应和PE38诱导产生的中和抗体毒性不容忽视,这也导致其只能在低剂量下使用(<0.8 mg/kg)。第二代PE片段mPE24显示出较低的脱靶效应和较强的肿瘤抑制作用。Wang等[53]构建了HN3-mPE24和HN3-HN3-mPE24,前者具有更高的GPC3结合能力、更优的细胞毒性和更高的最大耐受剂量。在肝癌异种移植小鼠模型中,静脉注射HN3-mPE24可诱导肿瘤明显消退,延长生存期。然而,免疫毒素的免疫原性作用和相对较短的半衰期限制了其进一步的临床应用。为了克服这一缺点,FLEMING等[54]利用添加白蛋白结合域(ABD)的策略以延长免疫毒素半衰期,得到HN3-ABD-T20、HN3-ALB1-T20。与HN3-T20相比,HN3-ABD-T20血清保留时间延长了45倍(半衰期:5.5 h vs 7 min),仅需HN3-T20剂量的十分之一即可有效消退肿瘤。靶向GPC3的免疫毒素,在治疗肝癌方面显示出一定的作用,具有进一步研究的价值。
-
CAR-T细胞由患者来源的T细胞产生,经基因工程改造的T细胞扩增后,再输注到患者体内[55]。GPC3作为一种有潜在价值的细胞表面抗原,近年来被用于开发CAR-T细胞(图2)。WU等[56]报道了一种靶向GPC3的CAR-T细胞,能够在体外杀死GPC3阳性肝癌细胞,并在小鼠肝癌异种移植瘤模型中诱导肿瘤消退。与此同时,联合使用索拉非尼和靶向GPC3的CAR-T细胞也被证明是具有疗效的治疗策略。LI等[57]开发了能够同时靶向GPC3与表皮生长因子受体(EGFR)的CAR-T细胞,其在抑制肝癌生长方面显示出了更强的治疗效果。基于上述临床前研究成果,多项临床试验探索了GPC3 CAR-T细胞在肝癌治疗中的应用。一项I期临床研究(NCT02395250)报道了GPC3 CAR-T细胞在13例难治性或复发性GPC3阳性的中国肝细胞癌患者中应用,患者对CAR-T细胞治疗表现出较好的耐受性,初步分析表明,经清除淋巴细胞的预处理后,靶向GPC3的CAR-T细胞展现出一定的疗效。
然而,使用CAR-T细胞同样会产生副作用,包括肿瘤溶解综合征、细胞因子释放综合征、以及非肿瘤组织的在靶效应。这些副作用而非肿瘤本身,可能同样致命。目前,NK-92细胞已经发展到能以最小的毒性实现较优的疗效。基因修饰NK-92细胞的安全性和细胞毒性特异性已在临床前试验中得到证实,这些细胞有潜力成为CAR的理想载体[58]。基于GPC3的CAR-T/NK细胞治疗策略有望为肝细胞癌免疫治疗提供新的手段。
-
在过去的几年中,研究人员开发了许多基于GPC3的基因治疗策略,包括使用miRNAs、短发卡样RNAs(shRNAs)和siRNAs等分子干预GPC3表达水平进行治疗(图2)。靶向GPC3的miRNA能以GPC3依赖的方式抑制肝癌细胞的生长,并诱导其凋亡[59]。使用GPC3 shRNAs进行RNA干扰,可以抑制肝癌细胞、肝癌异种移植瘤的增殖、侵袭[60];诱导细胞周期阻滞于G1期并引发细胞凋亡[61]。此外,利用siRNA敲低GPC3表达水平,可通过下调YAP,抑制肝癌细胞增殖并诱导细胞凋亡[62]。然而,尽管siRNA技术能实现单个基因的特异性沉默,但该技术因依赖于高特异性、低毒性的递送策略而很难应用于临床环境。为了克服siRNA的递送问题,WANG等[63]构建了一种纳米载体(NP-siRNA-GPC3 Ab),该载体在体外显示出抗肿瘤功效,未显示出明显的细胞毒性,并显著抑制小鼠原位肝癌异种移植肿瘤的生长。虽然这些基于基因治疗的技术在体外和体内显示出了一定的疗效,但在应用于临床研究前仍需进一步的探索。
-
抗GPC3抗体和免疫检查点抑制剂的联用,也是一种针对肝细胞癌的有效治疗方案(图2)。在过表达GPC3的小鼠肝脏肿瘤模型中,抗小鼠GPC3单克隆抗体与抗小鼠PD-L1抗体联用时,显示出更强的抗肿瘤活性[64]。与此同时,联合治疗的I期临床试验目前正在进行中,GC33和atezolizumab联合治疗显示出良好的耐受性,并在晚期、高表达GPC3、经既往治疗的肝细胞癌患者中显示出有效的肿瘤抑制作用[65]。因此,该策略较其他治疗方案可能具有更高的抗肿瘤疗效与安全性,可能具有治愈HCC患者的潜力。
-
光动力疗法(PDT)是一种治疗肿瘤的新方法,这种方法依赖于光敏剂产生活性氧,诱导肿瘤细胞凋亡,这种凋亡作用与肿瘤血管关闭和免疫活性增强有关,但其应用受到可见光组织穿透力差的限制,使用近红外(NIR)光敏剂可以解决这些限制(图2)[38]。例如,FENG等人将单克隆抗体与光敏性酞菁苷染料IR700偶联,通过近红外光照射靶向癌细胞。使用IR700-YP7和IR700-HN3进行的光免疫治疗在荷瘤小鼠中显示出治疗效果。此外,IR700-YP7联合蛋白结合型紫杉醇进行光免疫治疗,可显著增加蛋白结合型紫杉醇的递送量,提高治疗效果[66]。UCNPs@mSiO2-Ce6-GPC3纳米颗粒具有较好的生物相容性,毒性低,产生抗肿瘤作用的同时还可实现细胞成像[67]。半乳糖-金纳米棒是一种新型的多功能纳米结构,其包载GPC3 siRNA(siGPC3),可以同时实现GPC3基因沉默效应和光热治疗作用,并且能用于癌症的协同治疗[68]。
-
经过20多年的研究,GPC3因与Wnt、Hh、YAP等肝癌相关信号通路的密切关系,使其从单纯的肝细胞癌生物标志物,逐渐演变为抗肝癌治疗的热门研究靶点。研究人员围绕GPC3这一靶点开发了单克隆抗体、双特异性抗体、肿瘤疫苗、CAR-T细胞等一系列治疗策略,其在临床前及临床研究中都显示出了一定的价值,但仍存在着疗效不佳、特异性不够或毒副作用等缺陷。同时,针对GPC3的蛋白功能、转录调控、转录后修饰等方面的研究仍存在着许多未解之谜。因此,进一步阐明GPC3在肝细胞癌的发生发展中所发挥的功能,并结合其它抗肿瘤药物研究策略:例如开发针对GPC3的小分子抑制剂,调控GPC3蛋白活性,或利用近年来较为热门的靶向蛋白降解技术,对GPC3表达水平进行高效干预,获得比目前可用的GPC3靶向药物,具有更强肿瘤抑制活性的药物,仍将是未来极具价值的研究方向。
Research Progress on Glypican-3 Targeted Therapy in Hepatocellular Carcinoma
doi: 10.12206/j.issn.2097-2024.202307047
- Received Date: 2023-07-21
- Rev Recd Date: 2023-08-15
- Available Online: 2023-10-23
- Publish Date: 2023-10-25
-
Key words:
- Hepatocellular carcinoma /
- Glypican-3 /
- signaling pathway /
- HCC therapy
Abstract: Liver cancer is the second leading cause of cancer-related deaths worldwide, with hepatocellular carcinoma (HCC) being the most common form of primary liver cancer. Glypican-3 (GPC3) is a cell membrane proteoglycan which is rarely expressed in normal adult tissues but is specifically upregulated in HCC, which makes GPC3 a reliable target for the diagnosis and treatment of HCC. The role of GPC3 in the regulation of cancer development through Wnt, YAP, hedgehog and other signaling pathways were reviewed in this article. GPC3-targeted therapies, such as monoclonal antibodies, bispecific antibodies, tumor vaccines, immunotoxins, CAR-T cells, and photosensitizer therapy were also summarized. These treatment methods offered promising approaches for HCC treatment and future treatment strategies for HCC based on GPC3 were prospected in this paper.
| Citation: | FANG Yuxin, LI Yu, LIU Baoshu, DONG Guoqiang. Research Progress on Glypican-3 Targeted Therapy in Hepatocellular Carcinoma[J]. Journal of Pharmaceutical Practice and Service, 2023, 41(10): 586-593. doi: 10.12206/j.issn.2097-2024.202307047 |








 DownLoad:
DownLoad: