-
聚合物囊泡(PSs) 是由两亲嵌段共聚物自组装形成的囊泡状“壳-核”结构微纳米载体,其结构类似脂质体(liposomes)[1],常用的聚合物材料包括聚乙烯醇(PVA)、聚乙二醇(PEG)、聚己内酯(PCL)、聚乳酸(PLA)、聚乳酸-羟基乙酸共聚体(PLGA)等,作为生物膜模型、药物控释和基因转染的载体而被广泛研究。脂质体制备过程中,其稳定性受磷脂酰胆碱和胆固醇的比例、温度和pH等因素的影响,一定范围内磷脂酰胆碱含量高,高温和酸性环境都会破坏脂质体的稳定性。聚合物囊泡具有更加稳定和可控的结构特点,熵值较小,比脂质和表面活性剂更稳定,在化学修饰、生物分子偶联和物理结构等方面具有更好的稳定性和灵活性[2, 3],对(113.7±18.5)nm水的渗透性较低,可抵抗更大的压力[4]。聚合物囊泡既可以携带水溶性药物 (如氨基酸、多肽、蛋白类药物),将药物包封在内水相内,也可以携带脂溶性药物,将药物增溶在膜层中。根据聚合物的链长和分子量差异得到不同膜层厚度的聚合物囊泡,可提高疏水性药物在囊泡中的包封率[5]。聚合物囊泡的特殊结构和性质决定其可用于生物膜模拟、药物释放、催化、提供反应的微环境等,因此,在分子封装和控释领域有很大的潜在应用价值。作为药物载体,具有改变药物在体内的分布、防止药物降解失活、延长作用时间以及降低毒副作用等特点[6, 7]。
聚合物囊泡的膜稳定性好,但用于药物包载时可能影响药物的释放速率,达到药物作用有效浓度时间长,影响治疗效果[8]。不同嵌段聚合物制备的聚合物囊泡具有不同的膜厚度以及渗透特性。H+分子小、极性强、对囊泡膜的渗透性弱,Wu等[6, 9]的研究将H+的透膜性能作为考察囊泡膜壁的指标,通过考察H+的跨膜特性评估囊泡膜的渗透性,进一步模拟研究囊泡内外可否形成H+跨膜梯度,为囊泡的主动载药提供依据,同时发现1,4-二氧六环可对膜渗透特性进行调节,有利于药物包载。8-羟基-1,3,6-三磺酸芘钠盐(HPTS)是一种pH响应性的荧光探针,特定波长下的荧光强度随着溶液的pH值发生变化,可用于监测溶液的酸碱性。因此,本文采用H+跨膜转运考察聚合物囊泡的渗透性,对几种不同聚合物材料制备的囊泡和脂质体进行比较 ,以 pH敏感荧光探针HPTS对载药相关的透膜性能以及1,4-二氧六环对聚合物囊泡膜的透过性影响进行了考察[9],并与脂质体进行对比。
-
紫外分光光度仪(UV-2401PC,Shimadzu,日本),粒度/Zeta电位测量仪, (NICOMPTM 380 ZLS,NICOMP,美国),荧光分光光度仪(LS55 Luminescence spectroscopy,Perkin Elmer,英国),旋转蒸发仪(R-200,Buchi,德国),Satoris Mettler AE200天平(Satoris,德国),PHS-3TC pH计(上海天达仪器,中国),葡聚糖凝胶(Sepharose CL-4B, Pharmacia,瑞典),8-羟基-1,3,6-三磺酸芘钠盐(8-hydroxypyrene-1,3,6-trisulfonic acid trisodium salt,HPTS,Acros,美国),大豆卵磷脂(上海爱康精细化工有限公司,中国),胆固醇(上海如吉生物科技发展有限公司,中国),MPEG-PLGA(实验室自合成),PBD-b-PEO(聚氧乙烯-聚丁二烯,Polymer Source, 加拿大), PS-b-PEO (聚氧乙烯-聚苯乙烯,复旦大学高分子科学系黄骏廉教授惠赠),1,4-二氧六环(1,4-dioxane,AR,上海诺泰化工有限公司),其余试剂均为分析纯(中国医药集团上海化学试剂公司,中国)。
-
以开环聚合法合成PEG-PLGA,将20 mg PEG-PLGA溶于2 ml丙酮,缓慢注入PBS缓冲液(0.01 mol/L)中自组装制备聚合物囊泡(PSs-PLGA),超滤浓缩备用。薄膜分散法制备脂质体及两种聚合物囊泡(PBD-b-PEO、PS-b-PEO),将15 mg大豆磷脂与5 mg胆固醇混合溶于10 ml二氯甲烷中,20 mg PBD-b-PEO、20 mg PS-b-PEO分别溶于10 ml二氯甲烷中,3种载体材料分别采用旋转挥发法成膜,减压干燥24 h后用PBS缓冲液(0.01 mol/L)水化,冰浴探头超声得到脂质体、PSs-PBD和PSs-PS,超滤浓缩备用[10]。
-
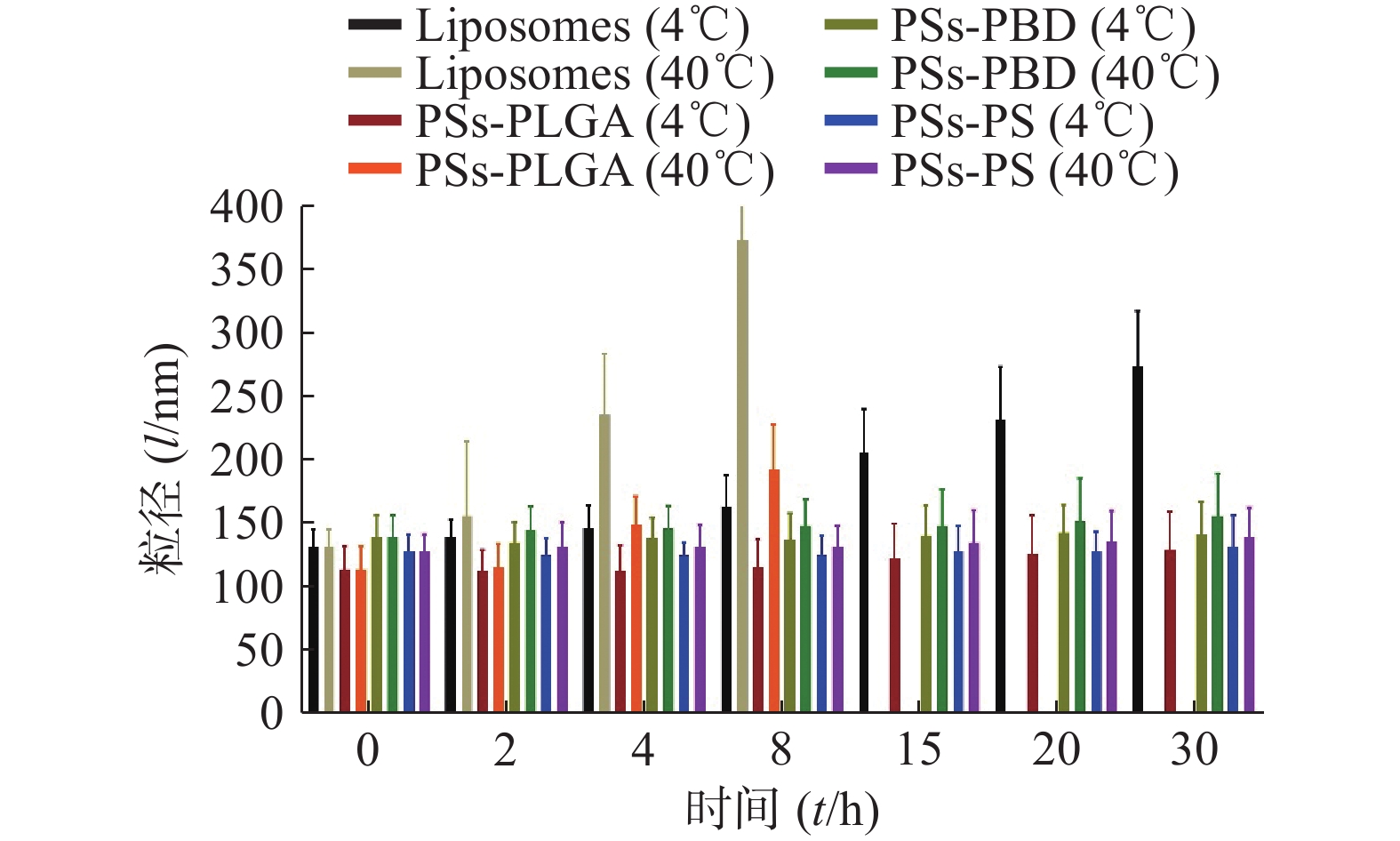
取适量已制备的脂质体和3种聚合物囊泡,在4 ℃恒温箱内静置,测量并记录时间t为0、2、4、8、15、20、30 d时的粒径,观察溶液分层和沉淀情况。同上,另取一组上述溶液,在40 ℃恒温箱内静置,重复上述实验。
-
在聚合物囊泡对药物的包载释放中,囊泡膜壁的组成成分和渗透性起着重要作用。研究者将H+的透膜性能作为考察囊泡膜壁的指标,研究H+的跨膜转运可用于囊泡膜的渗透性研究。本文采用H+跨膜渗透考察囊泡的膜渗透性,对几种不同聚合物材料制备的囊泡和脂质体做出对比。
-
HPTS是pH响应性的荧光探针,荧光强度随着溶液pH值变化而发生变化,用于监测溶液的酸碱性。HPTS的激发波长(λex)分别是403 nm和454 nm,发射波长(λem)是 509 nm,激发波长的强度之比(I454/I403)与HPTS溶液体系的pH相关,而发射波长与HPTS溶液体系的pH无关。根据这些性质,HPTS作为pH敏感的荧光探针可用于测定聚合物囊泡外水相的pH值变化[6]。
配制一系列不同pH值梯度的缓冲溶液,分别加入适量HPTS(浓度为2.5 µmol/L),检测并记录激发光波长在403 nm和454 nm的荧光强度I403和I454,分别将I403/I454和I454/I403与pH值按Boltzmann函数进行曲线拟合,得到I403/I454和I454/I403与pH值的相关关系。
-
制备聚合物囊泡和脂质体,方法同“1.2”项。用0.2 mol/L的柠檬酸缓冲溶液(pH值 4.0)代替pH值7.4的PBS溶液(0.01 mol/L)。将聚合物囊泡或脂质体过Sepharose CL-4B柱,生理盐水洗脱。将上述聚合物囊泡或脂质体混悬液中加入适量HPTS(浓度为2.5 µmol/L),检测并记录激发光波长在403 nm和454 nm的荧光强度I403和I454。根据I454/I403与pH值的相关性计算囊泡外水相的pH值与H+浓度。
-
根据Fick’s第一定律,H+从单个聚合物囊泡扩散到外水相的速率为:
式中,M为H+由聚合物囊泡传播至外水相的量,A为聚合物囊泡的表面积,K为H+在聚已内酯(囊泡的膜层)与水中的分配系数,D为H+的传播系数,L为聚合物囊泡的膜厚,△C为囊泡的内水相H+浓度(Cin)和外水相H+浓度(Cout)之差。因此,囊泡外水相中H+浓度为:
式中,Vin为囊泡内腔体积,Vout为外水相体积。单个囊泡内腔体积Vin根据公式
$ {V_{in}} = 4/3 \text{π} {(D/2 - L)^3} $ 计算,其中,D为囊泡的粒径的平均数。由于K值未知,所以可用表观扩散系数D*代替DK。而由于Cin为定值0.1 mmol/L,因此方程(2)可转化为:参考文献[9]进行推导,同时囊泡外水相中H+浓度与时间可按方程
$ {C_{out}} + {C_{in}} = A\sqrt {t + B} $ 拟合,其中:由于实验中t(s)>>B,表明聚合物囊泡外水相中H+浓度(pH值)与t1/2成正比,因此方程(3)最终可转化为:
将聚合物囊泡外水相中H+浓度(pH值)和时间按方程(4)拟合后,可求算H+在聚合物囊泡跨膜转运过程中的表观扩散系数D*。
-
嵌段共聚物在溶液中自组装形成聚合物囊泡的行为与共聚物疏水链段与亲水链段的比例有关,当亲水端比例为20%~40%时为囊泡的形成区域。PEG与PLGA以25∶75(w/w)的比例合成PEG-PLGA,以溶剂注入法在溶液中自组装制备PSs-PLGA,囊泡呈明显的圆形囊壁状结构,外壁厚度d约为10 nm,粒径为(113.7±18.5)nm [10, 11]。以薄膜挥发法制备了PSs-PBD和PSs-PS及脂质体,粒径分别为(139.2±13.1)、(128.0±12.54)、(132.1 ±10.5)nm。
-
粒径稳定性试验结果见图1,与脂质体相比,3种聚合物囊泡在4 ℃放置的粒径变化均更小,不易出现沉淀和分层,而且随着放置时间的延长,这种趋势仍保持,说明聚合物囊泡相较脂质体更稳定。随着时间延长,在40 ℃条件下,脂质体出现结构的破坏无法测定粒径,PSs-PLGA也逐渐显示结构的破坏,而PSs-PBD和PSs-PS的粒径相对保持较好,聚合物囊泡的结构稳定性优于脂质体。
-
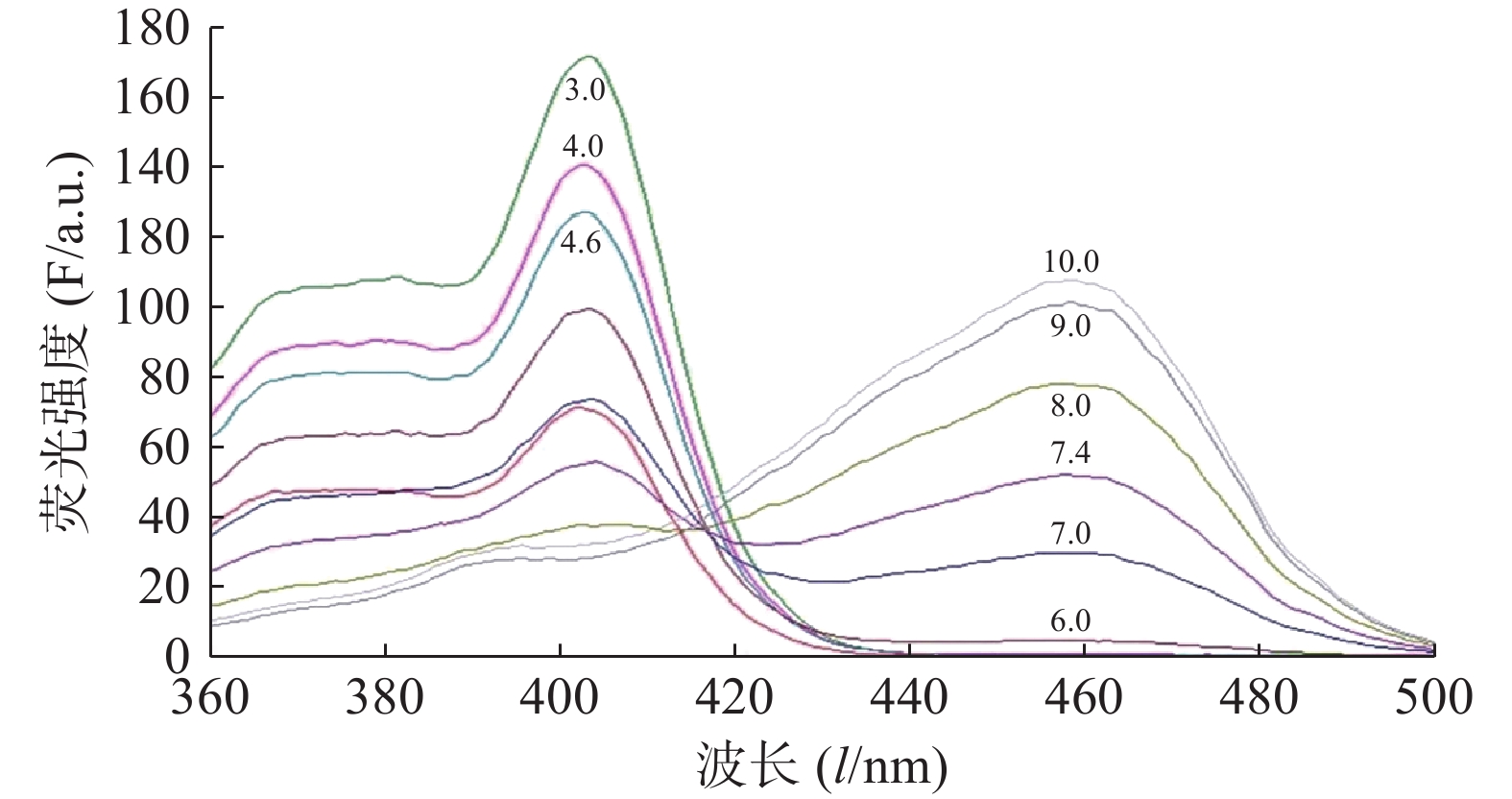
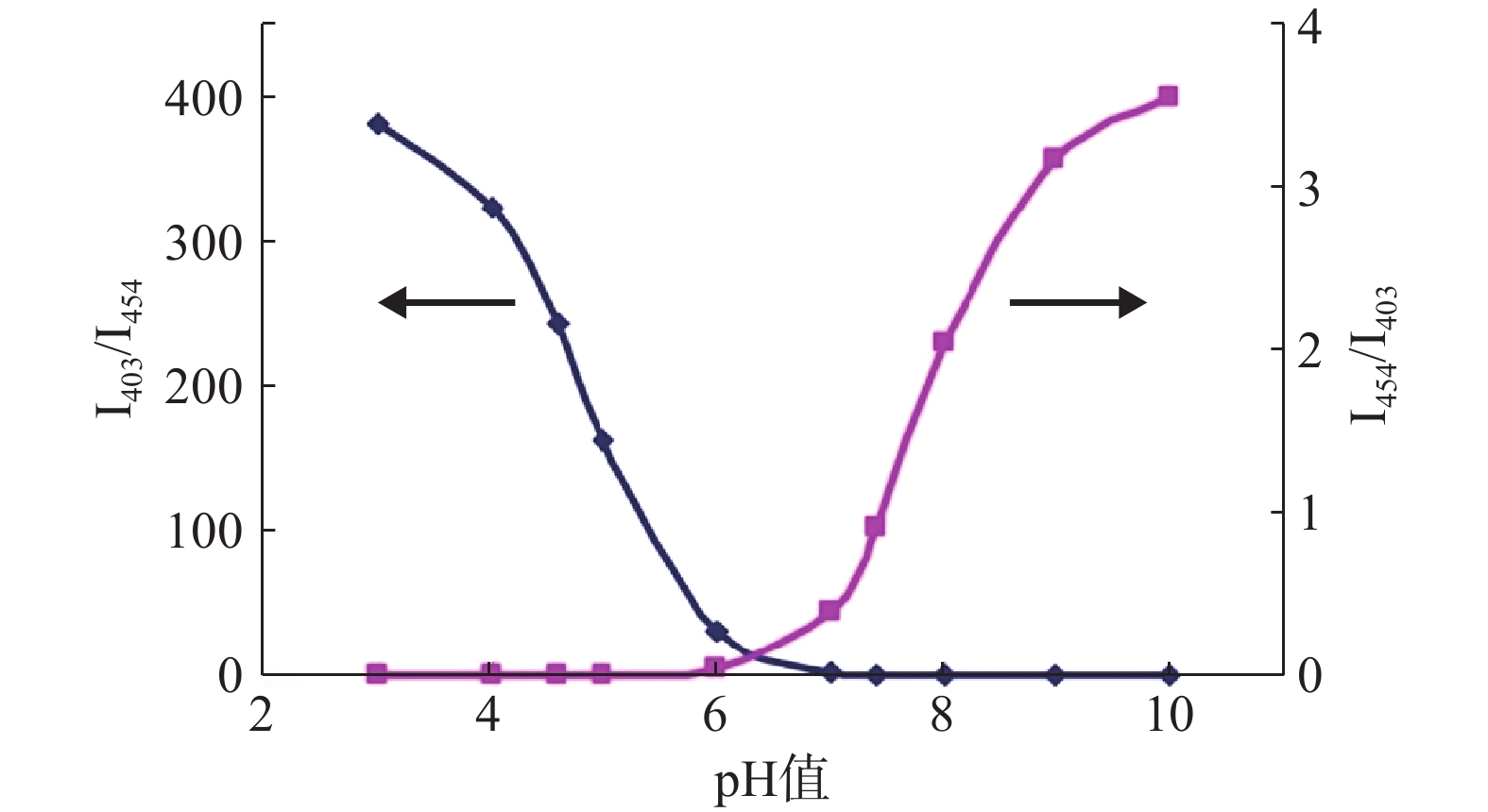
图2为HPTS在不同pH值的缓冲液中的荧光激发光谱图。如图2所示,HPTS的荧光激发光光谱有明显的pH值依赖性。pH 值10.0时,HPTS在454 nm的荧光强度最高,而在403 nm荧光强度最低,表明HPTS的大部分-OH离子化。随着pH的降低,HPTS的-O-质子化逐渐增多,HPTS在454 nm的荧光强度逐渐降低,而在403 nm的荧光强度逐渐升高。当pH 值< 6.0,454 nm峰基本消失,而403 nm峰强度随着pH值的降低继续逐渐升高。
将I454/I403或I403/I454对pH值作图,如图3所示,I454/I403随pH值升高而增加。将I454/I403或I403/I454对pH值按Boltzmann函数回归,即可得到I454/I403或I403/I454与pH值的相关关系。在特定pH范围内,可通过I403/I454与pH值的关系来测定聚合物囊泡外水相pH值。
I454/I403与pH值:
$ y = \displaystyle\frac{{5.236 + (0.032 - 1.548)}}{{1 + \exp \left[ {(x - 6.565)/0.473} \right]}} $ ,R=0.997I403/I454与pH值:
$ y = \displaystyle \frac{{0.08107 + (406.852 - 0.147)}}{{1 + \exp \left[ {(x - 4.265)/0.323} \right]}} $ ,R=0.995 -
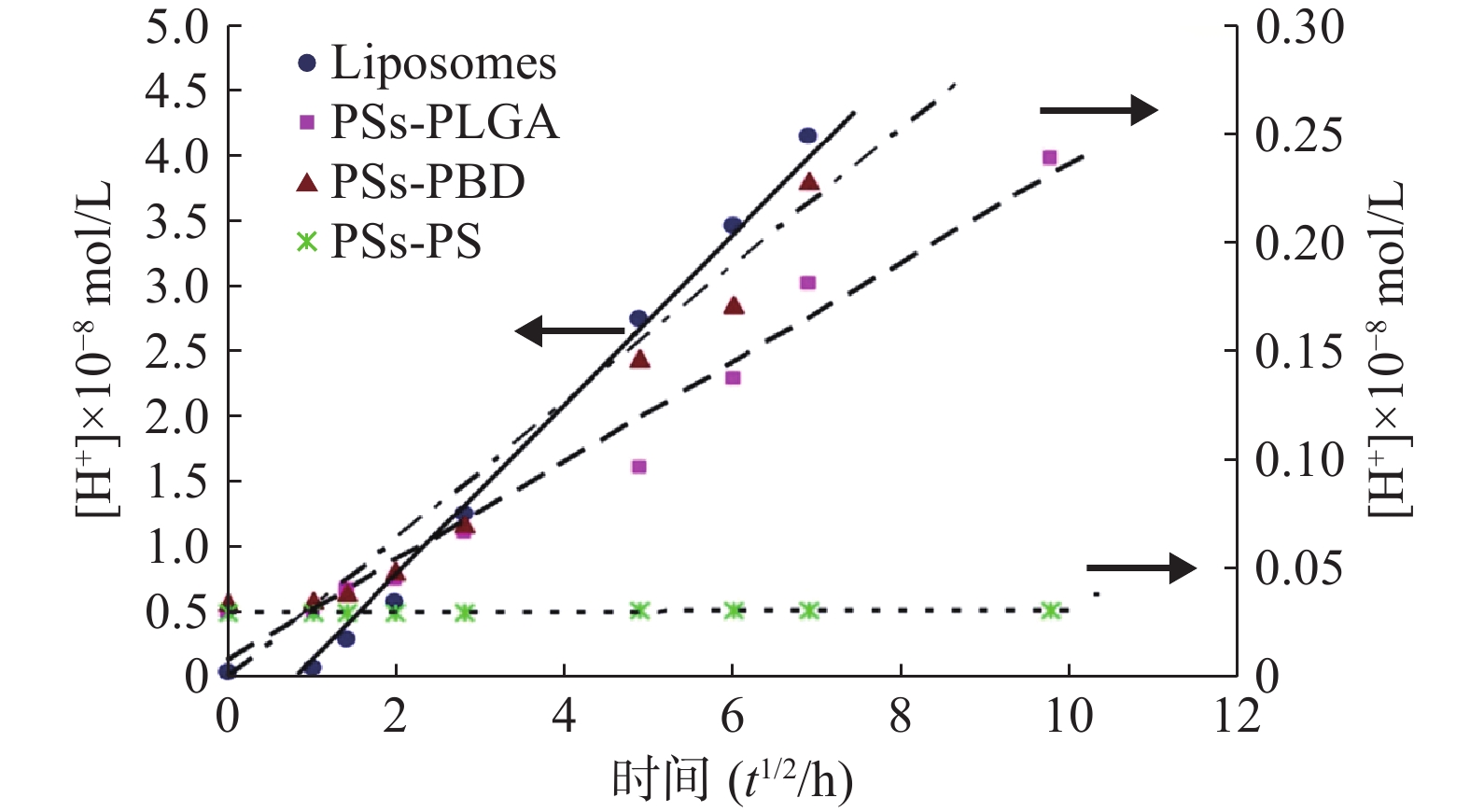
聚合物囊泡或脂质体外水相pH值测定结果见表1。将囊泡外水相中H+浓度[H+](Y)对t1/2(X)作图(图4),结果表明囊泡外水相中H+浓度与t1/2呈线性相关。
时间
(t/h)Liposomes PSs-PLGA PSs-PBD PSs-PS pH值 [H+]×
10−8 mol/LpH值 [H+]×
10−8 mol/LpH值 [H+]×
10−8 mol/LpH值 [H+]×
10−8 mol/L0 9.27±0.08 0.054±0.002 9.52±0.06 0.030±0.001 9.46±0.02 0.035±0.001 9.52±0.03 0.030±0.001 1 9.14±0.08 0.072±0.001 9.52±0.02 0.030±0.003 9.44±0.05 0.036±0.002 9.52±0.07 0.030±0.001 2 8.52±0.11 0.302±0.015 9.39±0.06 0.041±0.002 9.40±0.06 0.040±0.004 9.52±0.03 0.030±0.001 4 8.23±0.07 0.589±0.005 9.34±0.14 0.046±0.003 9.31±0.15 0.049±0.001 9.52±0.05 0.030±0.002 8 7.90±0.07 1.259±0.004 9.17±0.08 0.068±0.002 9.15±0.13 0.071±0.006 9.52±0.04 0.030±0.003 24 7.56±0.04 2.754±0.011 9.01±0.08 0.098±0.003 8.83±0.22 0.148±0.007 9.52±0.05 0.031±0.003 36 7.46±0.09 3.467±0.013 8.86±0.12 0.138±0.003 8.79±0.16 0.162±0.006 9.52±0.05 0.031±0.001 48 7.38±0.14 4.169±0.015 8.74±0.10 0.182±0.002 8.64±0.09 0.229±0.005 9.51±0.04 0.031±0.001 96 — — 8.62±0.08 0.240±0.001 8.49±0.05 0.324±0.007 9.50±0.07 0.032±0.002 注:“—”表示未检测。 脂质体:Y = 0.6464X-0.4423(R= 0.989);PSs -PLGA:Y = 0.0288X + 0.0087 (R = 0.984);PSs-PBD:Y = 0.0311X + 0.001(R = 0.983);PSs-PS:Y = 0.0002X + 0.03(R = 0.958)
从回归方程的斜率可知,脂质体中H+的释放速度远远大于囊泡中H+的释放,而3种聚合物囊泡相比,PSs-PLGA的释放速率大于PSs-PS,与PSs-PBD没有显著差异,PSs-PS在测定时间内几乎没有H+透膜现象发生,这与材料的属性密切相关。将上述脂质体和PSs -PLGA两直线的斜率代入方程(10):
求得H+的表观扩散系数。其中Cin = 0.1 mmol/L,Vout =2 ml,计算结果见表2。结果表明,H+在脂质体的表观扩散系数D*=8.39×10−4 cm2/s,是H+在PSs-PLGA的表观扩散系数D*=3.51×10−8 cm2/s的2.39×104倍。囊泡膜壁对H+的渗透性远远低于脂质体。这一性质将既有利于囊泡内被包封药物的保存,又表明囊泡内外可以建立H+跨膜梯度,为囊泡的主动载药提供依据。
名称
粒径
(l/nm)Vin
(v/ml)A ×10−10
(s/cm2)N L
(l/nm)D*
(cm2/s)Liposomes 132.1 10.49×10−16 5.39 5.5×1015 3 8.39×10−4 PSs-PLGA 113.7 4.31×10−16 4.06 2.7×1015 10 3.51×10−8 PSs-PBD 139.2 8.01×10−16 6.08 1.6×1015 10 2.48×10−8 PSs-PS 128.0 2.48×10−16 5.14 1.02×1015 25 1.53×10−12 -
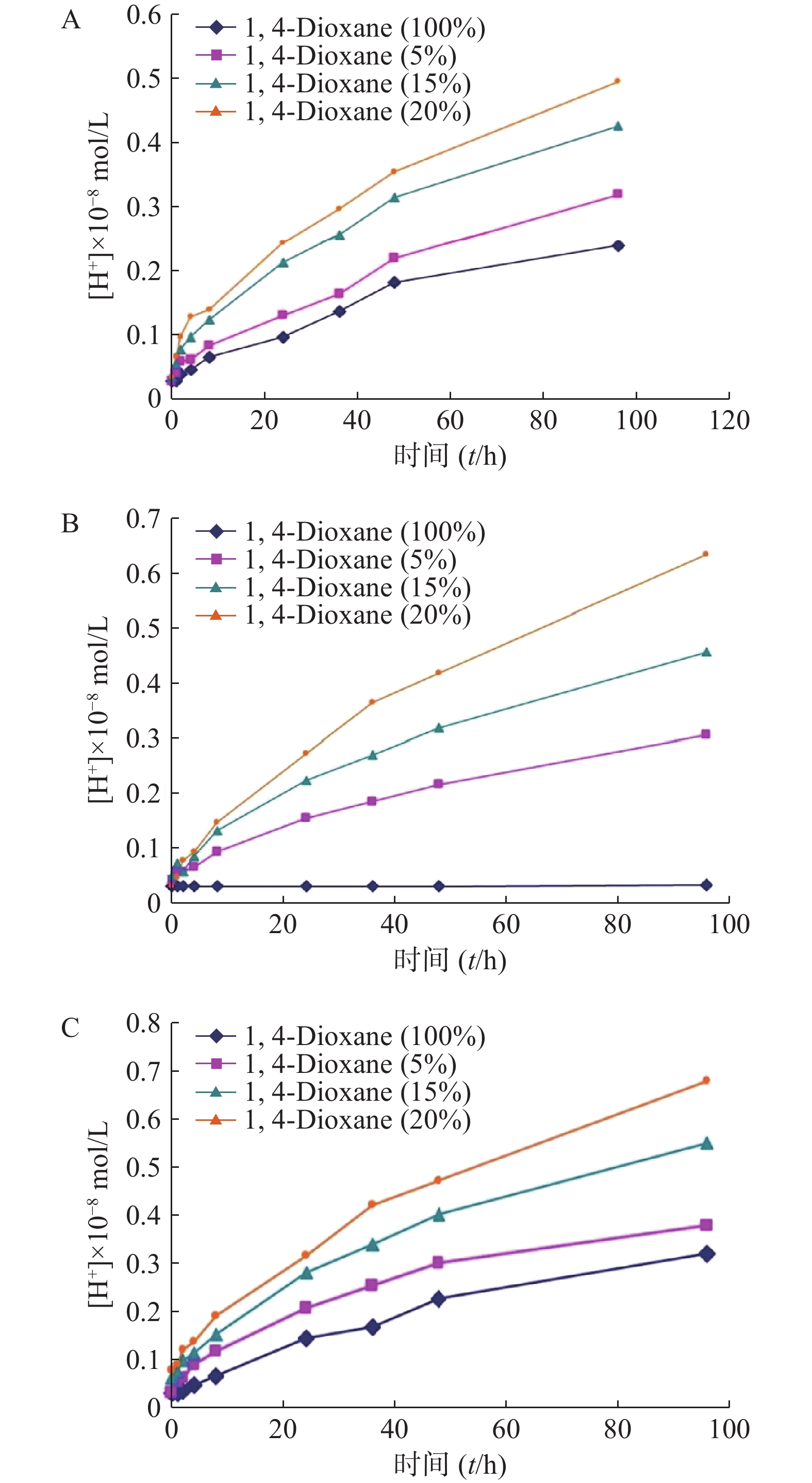
聚合物囊泡的膜壁较厚,而且分子间排列结构紧密,所以对分子的渗透性低。考察1,4-二氧六环对PEG-PLGA以及PBD-b-PEO,PS-b-PEO制备的囊泡的膜透过能力的影响。囊泡外水相加入1,4-二氧六环室温孵育,浓度分别是5%、15%、20%,按时间点测定H+浓度,测定结果见图5。随着囊泡外水相中1,4-二氧六环含量的增加,由5%~20%,囊泡膜层对H+的渗透能力加强。上述实验结果表明1,4-二氧六环对PEG-PLGA囊泡膜的渗透性具有很好的调节作用,其作用与囊泡外水相中的1,4-二氧六环含量成正相关(P<0.05)。1,4-二氧六环对聚合物囊泡的渗透能力有显著提高。
-
本研究采用溶液中自组装法制备PSs-PLGA,并与不同材料制备的聚合物囊泡以及脂质体进行稳定性及膜渗透特性的考察。HPTS是pH响应性的荧光探针,荧光强度随着溶液的pH值变化而发生变化,利用HPTS对pH的响应性,设计H+渗透实验评价聚合物囊泡的膜渗透性性能。结果表明,PSs-PLGA的膜渗透性较脂质体低,而比PBD-b-PEO和PS-b-PEO制备的囊泡膜渗透性高,适度的渗透性有利于囊泡中药物释放和装载。同时1,4-二氧六环可以作为PSs-PLGA的膜层增塑剂,调节膜的渗透性能,改善PSs-PLGA的载药和释药能力。FDA批准PLGA作为药用高分子材料,因其具有良好的生物降解和与分子量相关的缓释药物作用。作为一种与脂质体结构类似的纳米载体,与聚合物纳米粒不同,聚合物囊泡由于是中空壳核结构,因此其药物释放更快且载体的降解周期更短。兼具稳定性的优点,结合靶向配体的修饰以及聚合物材料合成策略,PLGA为基础的聚合物囊泡在药物靶向递送中具有广泛的研究价值[12]。
Study on the stability and H+ permeable membrane properties of polymersomes
doi: 10.12206/j.issn.2097-2024.202309010
- Received Date: 2023-09-06
- Rev Recd Date: 2023-10-10
- Available Online: 2024-01-19
- Publish Date: 2024-01-25
-
Key words:
- polymersomes /
- liposomes /
- nanocarriers /
- 1, 4-dioxane /
- permeability
Abstract:
| Citation: | BIAN Kangqing, GUO Lingyi, CHI Wenya, YU Yuan. Study on the stability and H+ permeable membrane properties of polymersomes[J]. Journal of Pharmaceutical Practice and Service, 2024, 42(1): 12-17. doi: 10.12206/j.issn.2097-2024.202309010 |








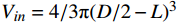
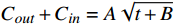
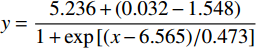
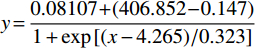
 DownLoad:
DownLoad: