-
肺型氧中毒是长期吸入高浓度氧气导致的肺部损伤,表现为肺组织充血、水肿、炎性浸润等,其中以多型核白细胞为主[1]。活化的多型核白细胞可以激活氧化酶,进而产生和释放大量的炎症介质和氧自由基,进一步介导级联式扩大的炎症反应对肺部造成损伤[2]。在饱和潜水或重型减压病患者进行加压治疗时,肺型氧中毒已成为最常见的并发症[3]。然而,目前关于高压氧(HBO)诱导的肺型氧中毒发生机制缺乏深入的探讨。
山莨菪碱(ANI)是一种毒蕈碱(M)受体阻滞剂,新斯的明(NEO)是一种临床常见的胆碱酯酶抑制剂。我们课题组前期研究发现ANI和NEO以500∶1的比例组成复方,简称新斯莨菪碱,能协同地激活α7nAChR,并减轻二者联用所致的不良反应。研究表明激活α7nAChR受体在感染性休克[4]、类风湿关节炎[5]、挤压综合征[6]、脑卒中[7]等动物模型上均发挥保护作用。然而,在HBO诱导的肺型氧中毒的发生、发展过程中,新斯莨菪碱是否可以用于肺型氧中毒的治疗?其治疗作用的病理生理机制是什么?这些都尚未阐明。
铁死亡是一种以细胞内铁依懒性的活性氧(ROS)异常增高,导致细胞内ROS的生成和降解失衡为特征的细胞死亡方式[8]。高浓度氧暴露条件下生成的大量ROS,一方面可直接对肺细胞产生损害,另一方面可参与炎症反应,促进炎症细胞渗透聚集,使各种致炎因子的表达增多,进一步加重肺的急性炎症反应[9]。
本文探究了新斯莨菪碱对HBO诱导的肺型氧中毒是否具有保护效应,并在此基础上,阐明铁死亡在新斯莨菪碱治疗HBO诱导的肺型氧中毒中的作用,以期为肺型氧中毒的临床防治提供新的理论指导和思路。
-
雄性C57BL/6小鼠,6~8周龄,小鼠购自上海斯莱克公司。所有小鼠均饲养于22℃,昼夜循环12 h的独立饲养系统中。小鼠自由饮食、自由饮水。所有实验均经海军军医大学实验动物伦理委员会批准并按照指南进行。
-
小型实验动物氧舱(RDC150-300-6,海军军医大学);台式高速冷冻离心(SCILOGEX);7500RT-PCR 仪器(Applied Biosystems 公司);激光共聚焦显微镜(日本Olympus公司);酶标仪(瑞士Tacan 公司);高速组织研磨仪(上海净信实业发展有限公司);Trizol、BCA 法蛋白定量试剂盒(美国GLPBIO公司);苏木精-伊红(上海Sangon Biotech公司);戊巴比妥(上海国药集团化学试剂公司);RT-PCR试剂盒(日本Takara公司);组织铁检测试剂盒(北京Solarbiog公司);伊文思蓝(美国Sigma-Aldrich公司);MDA、GSH检测试剂盒(上海Beyotime公司);Perls stain、抗谷胱甘肽过氧化物酶4(GPX4)抗体、抗β-actin抗体(英国Abcam公司);驴抗兔/驴抗鼠二抗、Odyssey 扫膜仪(LI-COR公司)。
-
将小鼠置于动物加压舱内,先用纯氧冲洗舱5 min,然后将压力在5 min内调至舱内为100% O2、2.5 ATA,维持6 h,在暴露过程中保持 0.5 L/min的连续氧流量,每1~2 h调整气阀进行快速通气,同时舱内放置碱石灰以避免呼吸产生的CO2在舱内蓄积。动物实验分组如下:①正常组:腹腔注射给予生理盐水10 ml/kg,常压饲养;②模型组:HBO暴露前30 min给予生理盐水10 ml/kg,2.5 ATA暴露6 h;③新斯莨菪碱组:HBO暴露前30 min给予ANI(25 mg/kg)+NEO(50 μg/kg),2.5 ATA 暴露6 h。
-
使用戊巴比妥钠麻醉并处死小鼠,解剖取小鼠左肺组织于4%多聚甲醛中固定,然后用石蜡包埋并切成5 μm的薄片。在 200倍光学显微镜下观察组织炎症、水肿或其他损伤。组织学评分:0分为正常组织学;1分代表轻度白细胞浸润和毛细血管充血;2分代表轻度白细胞浸润、血管周围水肿、肺结构部分破坏和出血;3分代表强烈的白细胞浸润和肺结构破坏。
-
HBO暴露6 h后,将小鼠固定,尾静脉注射1%伊文思蓝(50 mg/kg)后放回笼中自由活动,约30 min处死动物,用PBS灌流后取肺组织,用滤纸擦干肺组织表面水分并对肺组织进行拍照。每100 mg肺组织加入2 ml甲酰胺,55℃孵育18 h,反应结束后10 000×g 离心30 min后取上清液,并在630、740 nm处测量吸光度。
-
将小鼠麻醉处死后,取新鲜左肺组织,滤纸擦干表面血迹后称重记为湿重,随后将肺组织置于60℃干燥箱中,每日测量肺组织重量,直至肺组织重量不再变化,此时肺组织重量为干重。肺含水量(%)=(湿重−干重)/湿重×100%。
-
用1 ml预冷PBS经气管插管灌洗肺3次,收集混合的支气管肺泡灌洗液(BALF),将收集到的BALF以1 500 r/min 离心10 min获得其上清液,BCA法测定试剂盒定量BALF中总蛋白。
-
30 mg肺组织在冷PBS中清洗,在冰上用匀浆器在铁测定缓冲液中匀浆,在4℃以16 000×g离心10 min,收集上清液用铁检测试剂盒进行组织铁含量测定。
-
戊巴比妥钠麻醉处死小鼠,取新鲜肺组织于4%多聚甲醛中固定,固定48 h后进行梯度脱水,二甲苯置换,浸蜡;包埋、切片(厚度为5 μm)。将石蜡切片脱蜡后用Prussian Blue Stain(等体积的亚铁氰化钾溶液和盐酸溶液混合,制成铁染色工作液)染色15 min,洗涤,然后用核固红溶液染色5 min,洗涤,封片后在Olympus光学显微镜下观察染色切片。
-
取100 μg肺组织于300 μl PBS溶液中,匀浆机研磨后,以12 000 r/min离心10 min后取上清液待测。所有试剂盒均按照制造商的说明书使用,按检测工作液与上清2∶1配好检测体系后100℃水浴15 min,冷却后于532 nm处测吸光度。
-
取100 μg肺组织,与700 μl蛋白去除试剂S溶液混匀,匀浆机研磨后以12 000 r/min,离心10 min后取上清液进行总谷胱甘肽测定。所有试剂盒均按照制造商的说明书使用,25℃反应60 min即可测得氧化性谷胱甘肽(GSSG)含量,还原GSH含量=总谷胱甘肽含量−GSSG×2 。
-
动物出舱后,解剖取肺组织,使用TRIzol试剂提取组织总RNA并逆转录成cDNA,进行PCR扩增,采用2–ΔΔCT分析目的基因的相对表达量,引物序列见表1。
引物名称 引物序列(5′—3′) IL-6(F) CCAGAAACCGCTATGAAGTTCC IL-6(R) GTTGGGAGTGGTATCCTCTGTGA IL-1β(F) GTTCCCATTAGACAACTGCACTACAG IL-1β(R) GTCGTTGCTTGGTTCTCCTTGTA TNF-α(F) CCCCAAAGGGATGAGAAGTTC TNF-α(R) CCTCCACTTGGTGGTTTGCT GAPDH(F) GTATGACTCCACTCACGGCAAA GAPDH(R) GGTCTCGCTCCTGGAAGATG 注:F: 正向引物; R: 反向引物。 -
提取肺组织蛋白,BCA法测定蛋白浓度。十二烷基硫酸钠-聚丙烯酰胺凝胶(SDS-PAGE)电泳分离蛋白质,然后转移到硝酸纤维素膜上,与GPX4、β-肌动蛋白共孵育。最后,根据情况将膜与驴抗兔或驴抗鼠二抗孵育,使用Odyssey 扫膜仪获取图像,通过Image J 软件对条带进行定量分析。
-
实验数据的分析以及数据处理均使用GraphPad Prism 8.0 软件进行。所有值以(
$ \bar{x}\pm s $ )表示,多组间比较用单因素方差分析(ANOVA)并 Bonferroni 检验,P<0.05 视为差异具有统计学意义。 -
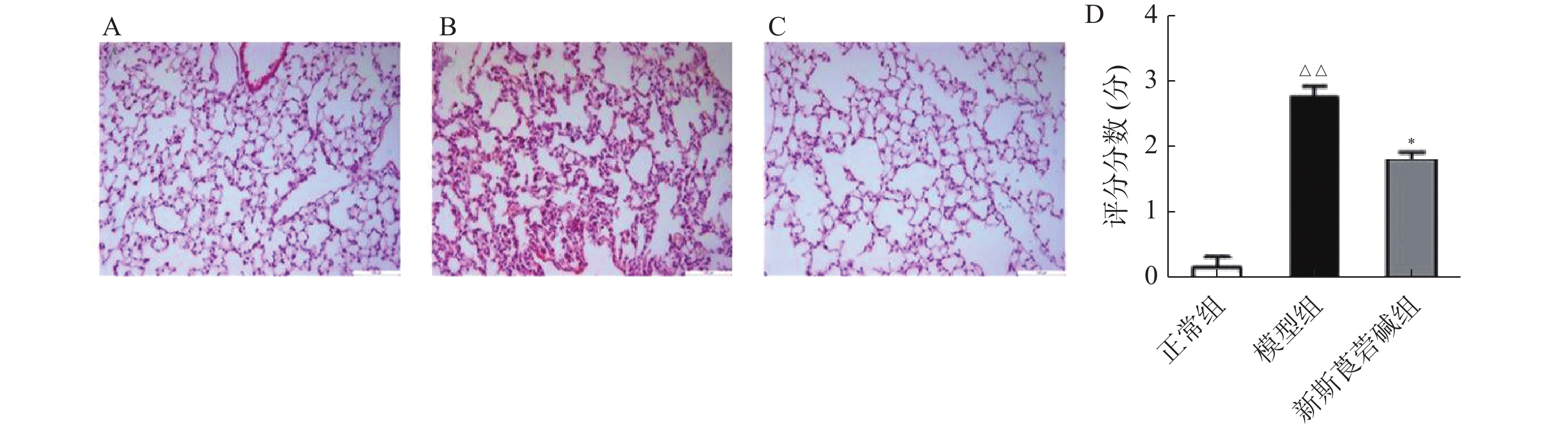
首先,探究HBO暴露后对肺组织的病理损伤情况以及新斯莨菪碱对HBO诱导的肺损伤的作用。将C57BL/6小鼠置于2.5 ATA的高压氧舱中暴露6 h建立肺型氧中毒模型,然后通过HE染色观察肺组织结构变化,如图1所示。正常组小鼠肺泡肺泡壁结构完整,间隔无增厚,肺泡腔内无渗出物;HBO暴露后,肺泡间隔出现水肿、增厚,肺泡腔内可见红细胞渗出,肺间质见大量炎性细胞浸润(图1B);给予新斯莨菪碱治疗后,肺水肿明显减轻,肺泡内炎性细胞浸润以及红细胞渗出减少,肺组织病理损伤减轻(图1C~D)。以上结果表明新斯莨菪碱治疗能减轻肺型氧中毒后肺组织的病理损伤。
-
从图2可以看出,模型组伊文思蓝染色加深,表明HBO暴露后肺渗透性增加,而新斯莨菪碱治疗后上述情况明显改善(图2A~B)。与正常组相比,模型组肺组织湿干比重明显增加,而新斯莨菪碱治疗后能够显著降低肺湿干比(图2C)。此外,HBO暴露后肺泡灌洗液中蛋白含量升高,新斯莨菪碱治疗能够有效减少肺泡灌洗液中蛋白的渗出(图2D)。这些结果表明,新斯莨菪碱治疗能够有效改善肺型氧中毒后的肺水肿及肺组织渗出。
-
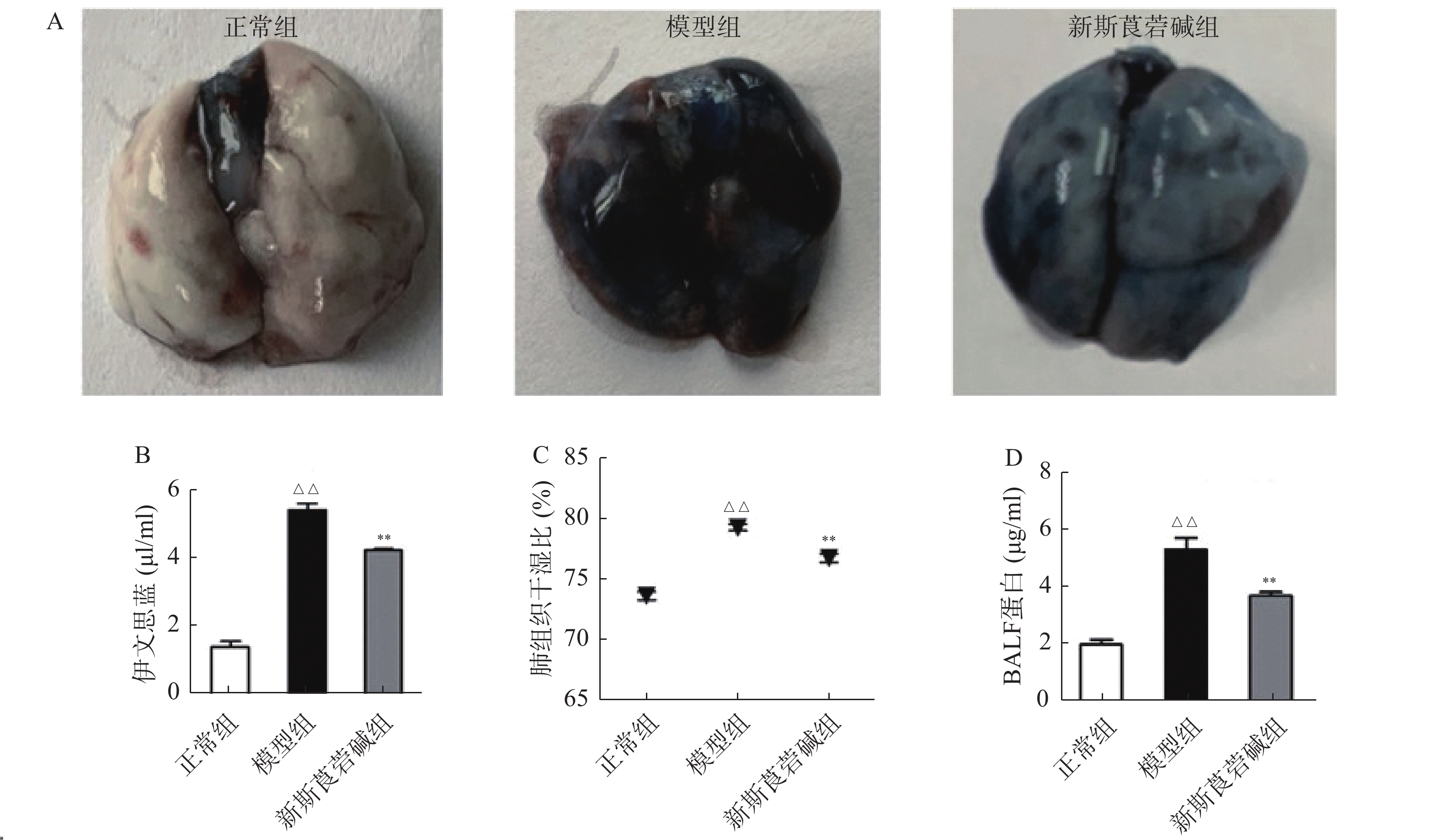
从图3中可以看出,HBO暴露后,肺组织中促细胞因子,如TNF-α、IL-1β、IL-6和IFN-γ的mRNA显著增高,抗炎因子IL-4及TGF-β则显著降低,而新斯莨菪碱能显著降低促炎因子的表达,同时提高抗炎因子的水平(图3A~F)。HBO暴露后肺组织氧化指标MDA升高,而抗氧化指标GSH降低,给予新斯莨菪碱治疗后,能明显逆转上述变化(图3G~H)。由此说明,新斯莨菪碱能降低HBO诱导的肺型氧中毒引起的炎症反应及氧化应激水平。
-
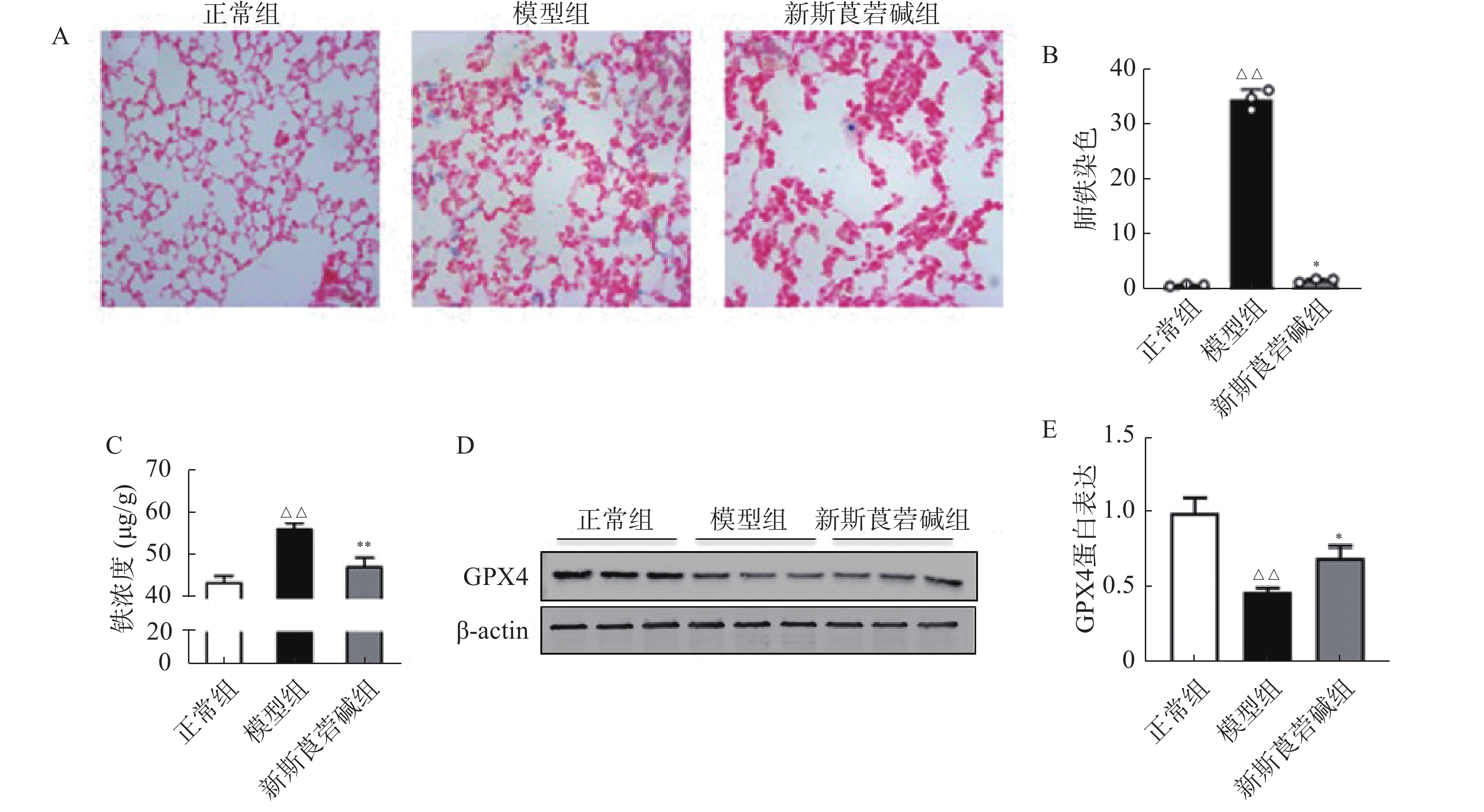
铁元素是维持正常生理活动所必需的,但过量的铁会产生对细胞有害的自由基。在病理状态下,铁蛋白的破坏导致细胞内游离铁的增高,脂质过氧化反应和游离铁的增高会导致细胞的铁死亡。如图4所示,HBO暴露后普鲁士蓝染色可见肺组织中铁含量增高,对肺组织铁离子含量进行检测得到了同样的结果(图4A~C)。GPX4是一种重要的抗氧化酶,HBO暴露后GPX4的表达降低(图4D~E),导致机体抗氧化应激能力减弱,对细胞有害的自由基无法被有效清除。而新斯莨菪碱治疗后可以改善GPX4的表达,降低肺组织铁含量,从而减少细胞铁死亡的发生,对肺组织起到保护作用。
-
HBO已被广泛用于多种疾病的治疗以及潜水作业中,并发挥了难以替代的作用[10]。然而,在饱和潜水或重型减压病患者加压治疗以及临床上用于治疗新生儿呼吸窘迫时,HBO诱导的肺损伤已成为最常见的并发症[11]。研究发现,当HBO环境持续存在时,将出现小气道功能障碍,表现为肺活量降低、气体扩散功能减弱和肺顺应性降低[12]。与之前的报道一致,本研究结果表明,与正常组相比,模型组的肺组织损伤显著加重;伊文思蓝染色显示HBO暴露后染色加深,肺湿/干比增加,同时肺泡灌洗液中的蛋白含量也显著增加,新斯莨菪碱治疗后这些变化被显著抑制,这些结果初步表明新斯莨菪碱与HBO诱导的肺型氧中毒的发生密切相关。
ANI属于传出自主神经M受体阻断剂,阻断M受体引起交感神经优势。NEO是一种可逆性胆碱酯酶抑制剂,可有效延长乙酰胆碱的作用时间,同时激活胆碱能抗炎通路[11]。Sun等[4]研究证实,两药物联合使用,不仅可以加强胆碱能抗炎通路的炎症抑制作用,还可降低NEO的不良反应,达到协同增强作用。本课题组前期的研究发现ANI和NEO联合使用,能够通过阻断巨噬细胞上的毒蕈碱型受体,使更多的乙酰胆碱作用于α7尼古丁乙酰胆碱受体,进而对胆碱能抗炎通路起到双向活化调节作用,进一步加强了对炎症反应的抑制作用[7]。本研究发现新斯莨菪碱能够显著降低肺组织中促炎因子TNF-α、IL-1β、IL-6和IFN-γ的表达。由此说明,新斯莨菪碱可减轻HBO导致的肺部炎症反应。
肺型氧中毒包括高压力损伤和高浓度氧损伤,目前认为ROS 及其代谢产物介导的生化紊乱是导致肺型氧中毒发生发展的主要因素。HBO环境下会产生大量氧自由基,可能导致细胞因子释放和炎症反应的增加,而异常的炎症反应可加剧肺损伤[13]。研究发现,HBO 暴露后肺组织中氧化指标MDA 显著升高、抗氧化指标 GSH 则显著降低,而给新斯莨菪碱治疗能明显改善肺组织氧化损伤情况。
铁死亡作为一种新发现的细胞死亡方式,其释放出内源性损伤相关分子(DAMPs)与炎症和氧化应激之间存在着诸多途径的交互作用。GPX4作为铁死亡的核心调控因子,近年来受到广泛关注。研究表明GPX4的缺乏或功能丧失会加剧铁死亡的发生,并促使细胞进一步受到氧化应激的影响[14]。Kang等[15]的研究发现在LPS诱导的脓毒症模型中,敲除GPX4导致脂质过氧化的加剧并进一步促进铁死亡的发生。本研究发现,HBO暴露后肺组织中铁含量增高、GPX4的表达量降低,而新斯莨菪碱治疗后可以逆转这些变化。
综上所述,新斯莨菪碱可能通过激活胆碱能抗炎通路,从而抑制炎症的和氧化应激的发生,进而减少了肺组织中游离铁的含量,最终抑制细胞铁死亡。下一步我们将通过体外实验,进一步验证在HBO诱导的肺型氧中毒模型中,新斯莨菪碱是否通过抑制铁死亡的产生从而减轻肺组织的损伤情况,以期为防治HBO诱导的肺型氧中毒提供新的策略。
Protective effect and mechanisms of neostigmine in combination with anisodamine against pulmonary oxygen toxicity
doi: 10.12206/j.issn.2097-2024.202310049
- Received Date: 2023-10-25
- Rev Recd Date: 2024-05-15
- Available Online: 2024-10-24
- Publish Date: 2024-10-25
-
Key words:
- hyperbaric oxygen /
- pulmonary oxygen toxicity /
- neoscopolamine /
- inflammation /
- oxidative stress
Abstract:
| Citation: | ZHANG Guangyu, DU Jing, LIU Mengzhen, ZHU Danni, YAN Hui, LIU Chong. Protective effect and mechanisms of neostigmine in combination with anisodamine against pulmonary oxygen toxicity[J]. Journal of Pharmaceutical Practice and Service, 2024, 42(10): 433-438, 444. doi: 10.12206/j.issn.2097-2024.202310049 |








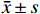
 DownLoad:
DownLoad: