-
在中医,脑卒中属于中风范畴,“气虚血瘀、痰瘀互结、毒损脑络”是中风的核心病机[1]。雷公藤系卫矛科雷公藤属藤本植物,又名震龙根、水莽子、断肠草、红紫根,以根茎入药,首载于《神农本草经》,味苦性寒,具有清热解毒、祛风通络、舒筋活血等功效,常用于免疫和炎性疾病的治疗。现代医学研究发现,炎症反应及缺血半暗带神经元凋亡在脑缺血再灌注损伤(CIRI)进展中发挥着重要作用,可作为防治CIRI新药研究的靶点[2-4]。雷公藤甲素为雷公藤的主要活性成分之一,化学结构属于二萜内酯类化合物,具有抗炎、抗凋亡等药理学作用[5-6]。Toll样受体4/核转录因子-κB(TLR4/NF-κB)通路是CIRI后炎症反应及神经元凋亡的重要调控机制[7]。有文献[8-9]报道雷公藤甲素能够抑制TLR4/NF-κB通路对大鼠类风湿关节炎、鼻炎具有一定抑制作用。然而,雷公藤甲素是否能够通过调控TLR4/NF-κB通路抑制CIRI仍需深入研究。因此,本实验将探讨雷公藤甲素对大鼠CIRI的影响及其机制,以期为雷公藤甲素应用于CIRI防治提供理论依据。
-
清洁级健康雄性7周龄Wistar大鼠144只,体质量210~240 g,购自杭州子源实验动物科技有限公司,实验动物生产许可证号SCXK(浙)2019-0004。饲养环境维持室温23~25 ℃、相对湿度45%~65%,自由饮水进食。动物实验遵守实验动物福利伦理审查指南。
-
雷公藤甲素(上海源叶生物科技有限公司,纯度≥98%);丁苯酞氯化钠注射液(恩必普药业有限公司,规格100 ml∶25 mg,国药准字H20100041);生理盐水(石家庄四药有限公司,规格500 ml,国药准字H13023200);注射用异戊巴比妥钠(上海上药新亚药业有限公司,规格0.1 g,国药准字H21021725);红四氮唑(TTC)、伊文思蓝(EB)(北京博奥森生物技术有限公司);4%多聚甲醛溶液(北京索莱宝生物技术公司);末端标记法(TUNEL)染色试剂盒和肿瘤坏死因子-α(TNF-α)、白细胞介素-1β(IL-1β)检测试剂盒(南京建成生物工程研究所);甲酰胺(天津科密欧化学试剂有限公司);TLR4、NF-κB、p-NF-κB、激活型半胱氨酸蛋白酶-3(cleaved Caspase-3)、B细胞淋巴瘤-2(Bcl-2)、Bcl-2相关X蛋白(Bax)、β-actin抗体和IgG二抗(北京博奥森生物技术有限公司);二喹啉甲酸法(BCA)蛋白浓度检测试剂盒、增强化学发光液(ECL)(武汉三鹰生物技术有限公司)。
-
TKY-BMB型石蜡包埋机(湖北康泰医疗设备有限公司);S7220型石蜡切片机(沈阳恒松科技有限公司);JY300C型电泳仪(美国Wealtec公司);Semi-Day型转膜仪(美国Bio-Rad公司);FluorChem HD2型凝胶成像仪(美国Protein Simple公司);BioTek Epoch型全波长酶标仪(美国伯腾仪器有限公司);BX53型显微镜(日本Olympus日立公司)。
-
将144只Wistar大鼠按照随机数字表法平均分为假手术组、模型组、雷公藤甲素低、中、高剂量组(0.2、0.4、0.8 mg/kg)[10]和丁苯酞组(6 mg/kg)[11],每组24只。造模前3 d开始1次/d腹腔注射(ip)给药,假手术组和模型组ip给予生理盐水,注射体积均为5 ml/kg。除假手术组外,其余5组均参照杨丽等[12]报道方法构建CIRI大鼠模型。
-
再灌注24 h后,分别随机取各组6只大鼠,参照文献[13]报道的方法进行神经功能缺失评分,无症状为0分、前肢不能伸展为1分、行走时转圈为2分、行走时跌倒为3分、不能行走或意识丧失为4分。ip戊巴比妥钠(40 mg/kg)进行麻醉后,颈椎脱臼处死,取大脑组织、-20 ℃冻存15 min后均匀厚度切为5片,2% TTC溶液恒温37 ℃避光染色30 min,每5 min翻转一次,正常组织呈红色、梗死组织呈苍白色,通过图像分析软件计算脑梗死率。
-
分别随机取各组6只大鼠,经尾静脉注射2% EB溶液4 ml/kg,30 min后实施麻醉,开胸后经“左心室-右心耳”灌注生理盐水至流出液清亮,取脑称重,1 ml/100 mg加入甲酰胺后60 ℃水浴24 h,4 ℃匀浆后15000 rpm离心20 min取上清液,通过酶标仪检测620 nm处吸光度值,对照标准曲线测定EB含量。
-
分别随机取各组6只大鼠,ip戊巴比妥钠(40 mg/kg)进行麻醉后,颈椎脱臼处死,取缺血侧大脑皮层组织,经4%多聚甲醛溶液固定、脱水、石蜡包埋、切片、烤片等处理后,按照HE试剂盒和TUNEL试剂盒操作说进行染色处理后,通过光学显微镜观察缺血半暗带皮层神经元病理学改变。显微镜下计数TUNEL染色切片5个视野内细胞数和凋亡细胞数,计数缺血半暗带皮层神经元凋亡率。
-
取各组剩余的6只大鼠,ip戊巴比妥钠(40 mg/kg)进行麻醉后,颈椎脱臼处死,剥取缺血侧大脑皮层组织。①取部分缺血侧大脑皮层组织,加入5倍量4 ℃生理盐水研磨匀浆,3 500 r/min离心5 min分离上清液,按照试剂盒操作说明通过ELISA法检测缺血侧大脑皮层组织TNF-α、IL-1β含量。②取剩余部分缺血侧大脑皮层组织,加入适量蛋白裂解液后研磨匀浆,冰上静置30 min使其充分裂解,4 ℃、12 000 r/min离心25 min分离上清液,检测总蛋白浓度并配平后,30 μg等量上样,经10 % SDS-PAGE凝胶电泳分离蛋白、转膜和5%脱脂牛奶封闭1.5 h后,加入一抗稀释液TLR4(1∶800)、NF-κB(1∶1000)、p-NF-κB(1∶1000)、cleaved Caspase-3(1∶800)、Bcl-2(1∶500)、Bax(1∶500)和内参β-actin(1∶1500)4 ℃避光孵育过夜,洗膜后二抗稀释液(1∶3 000)室温孵育1.5 h,洗膜后ECL显影,通过Image J软件分析蛋白条带灰度值。
-
运用SPSS 20.0软件进行数据统计分析,计量资料符合正态分布以(
$ \bar x \pm s $ )表示,多组间比较采用单因素方差分析,方差齐时两两比较采用LSD-t检验,方差不齐时两两比较采用Dunnett's T3检验,P<0.05为差异有统计学意义。 -
与假手术组比较,模型组大鼠神经功能缺失评分和脑梗死率显著升高(P<0.05)。与模型组比较,雷公藤甲素中、高剂量组和丁苯酞组神经功能缺失评分和脑梗死率显著降低(P<0.05)。与丁苯酞组比较,雷公藤甲素高剂量组神经功能缺失评分和脑梗死率显著降低(P<0.05)。见图1、表1。
组别 神经功能缺失
评分(分)脑梗死率
(%)EB含量
(μg/g)假手术组 0.00±0.00 0.00±0.00 0.49±0.06 模型组 2.84±0.39* 48.17±7.39* 1.54±0.27* 丁苯酞组 1.31±0.17△ 16.28±2.15△ 0.79±0.09△ 雷公藤甲素低剂量组 2.50±0.34 42.93±5.74 1.42±0.23 雷公藤甲素中剂量组 1.85±0.26△ 27.54±3.48△ 1.10±0.16△ 雷公藤甲素高剂量组 1.09±0.15△# 11.38±1.65△# 0.70±0.08△# *P<0.05,与假手术组比较;#P<0.05,与丁苯酞组比较;△P<0.05,与模型组比较。 -
与假手术组比较,模型组大鼠脑组织EB含量显著升高(P<0.05)。与模型组比较,雷公藤甲素中、高剂量组和丁苯酞组脑组织EB含量显著降低(P<0.05)。与丁苯酞组比较,雷公藤甲素高剂量组脑组织EB含量显著降低(P<0.05)。见表1。
-
假手术组大鼠皮层神经元呈圆形或椭圆形,形态饱满,着色均匀,胞核居中。模型组大鼠缺血半暗带皮层神经元呈现形态不规则,胞体萎缩呈空泡样变,着色较深,核膜边界不清,炎性细胞浸润等病理学改变。与模型组比较,雷公藤甲素各剂量组和丁苯酞组缺血半暗带皮层神经元明显改善,其中雷公藤甲素高剂量组效果优于雷公藤甲素低、中剂量组和丁苯酞组,见图2。
-
与假手术组比较,模型组大鼠缺血半暗带皮层神经元凋亡率显著升高(P<0.05)。与模型组比较,雷公藤甲素中、高剂量组和丁苯酞组凋亡率显著降低(P<0.05)。与丁苯酞组比较,雷公藤甲素高剂量组凋亡率显著降低(P<0.05),见表2。
组别 凋亡率(%) 假手术组 2.68±0.35 模型组 53.07±8.42* 丁苯酞组 17.69±2.90△ 雷公藤甲素低剂量组 45.93±7.67 雷公藤甲素中剂量组 31.52±5.08△ 雷公藤甲素高剂量组 12.88±1.74△# *P<0.05,与假手术组比较;#P<0.05,与丁苯酞组比较;△P<0.05,与模型组比较。 -
与假手术组比较,模型组缺血侧大脑皮层组织TNF-α、IL-1β含量显著升高(P<0.05)。与模型组比较,雷公藤甲素中、高剂量组和丁苯酞组TNF-α、IL-1β含量显著降低(P<0.05)。与丁苯酞组比较,雷公藤甲素高剂量组TNF-α、IL-1β含量显著降低(P<0.05)。见表3。
组别 TNF-α(ng/g) IL-1β(pg/g) 假手术组 1.41±0.22 37.06±4.81 模型组 2.76±0.35* 68.42±7.79* 丁苯酞组 1.85±0.23△ 46.93±5.62△ 雷公藤甲素低剂量组 2.54±0.32 63.81±6.54 雷公藤甲素中剂量组 2.07±0.25△ 55.74±5.91△ 雷公藤甲素高剂量组 1.62±0.21△# 40.27±5.39△# *P<0.05,与假手术组比较;#P<0.05,与丁苯酞组比较;△P<0.05,与模型组比较。 -
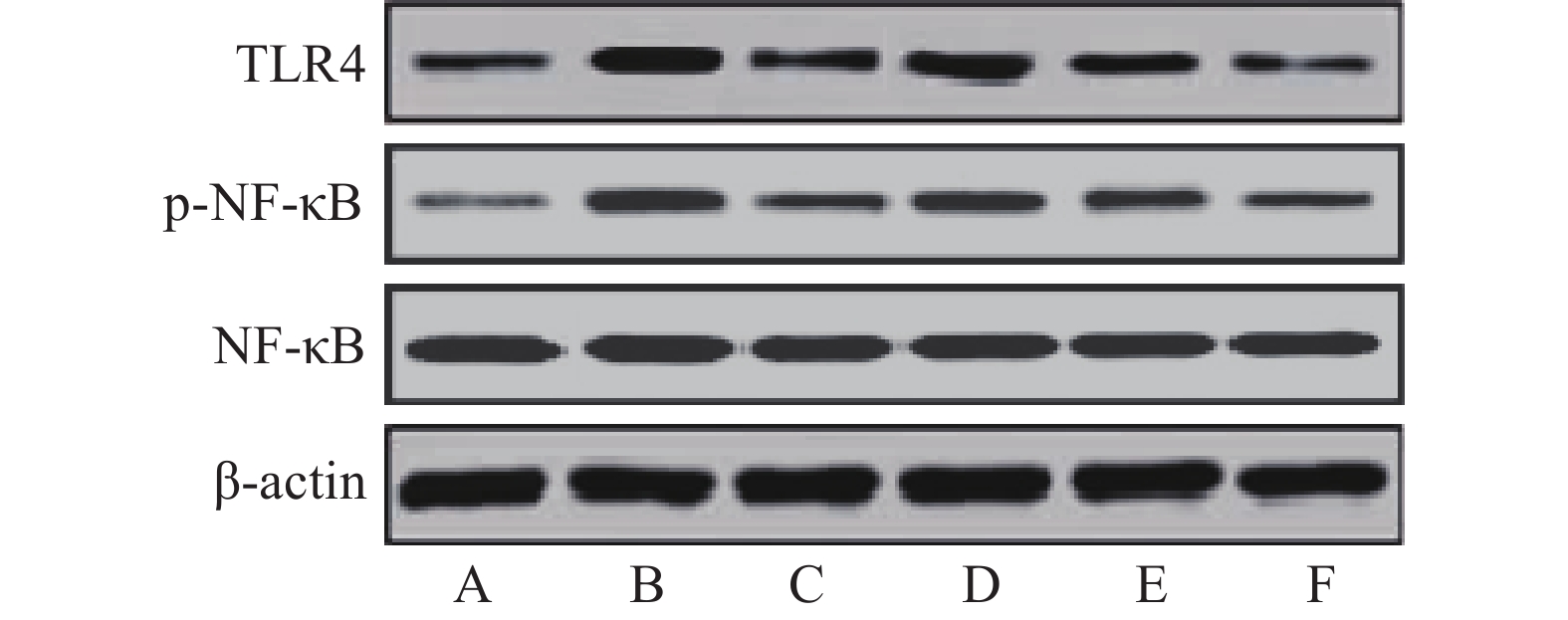
与假手术组比较,模型组大鼠缺血侧大脑皮层组织TLR4、p-NF-κB蛋白相对表达量和p-NF-κB/NF-κB比值显著升高(P<0.05)。与模型组比较,雷公藤甲素中、高剂量组和丁苯酞组TLR4、p-NF-κB蛋白相对表达量和p-NF-κB/NF-κB比值显著降低(P<0.05)。与丁苯酞组比较,雷公藤甲素高剂量组TLR4、p-NF-κB蛋白相对表达量和p-NF-κB/NF-κB比值显著降低(P<0.05),见图3、表4。
组别 TLR4/β-actin NF-κB/β-actin p-NF-κB/β-actin p-NF-κB/NF-κB 假手术组 0.08±0.02 0.83±0.15 0.10±0.02 0.12±0.03 模型组 1.03±0.21* 0.78±0.14 0.81±0.15* 1.04±0.22* 丁苯酞组 0.20±0.04△ 0.84±0.15 0.29±0.06△ 0.35±0.08△ 雷公藤甲素低剂量组 0.87±0.16 0.79±0.16 0.68±0.13 0.86±0.18 雷公藤甲素中剂量组 0.65±0.13△ 0.78±0.15 0.35±0.07△ 0.45±0.10△ 雷公藤甲素高剂量组 0.16±0.03△# 0.74±0.14 0.14±0.03△# 0.19±0.04△# *P<0.05,与假手术组比较;#P<0.05,与丁苯酞组比较;△P<0.05,与模型组比较。 -
与假手术组比较,模型组大鼠缺血侧大脑皮层组织cleaved Caspase-3、Bax蛋白相对表达量显著升高,Bcl-2相对表达量显著降低,Bax/Bcl-2比值显著升高(P<0.05)。与模型组比较,雷公藤甲素中、高剂量组和丁苯酞组cleaved Caspase-3、Bax相对表达量显著降低,Bcl-2相对表达量显著升高,Bax/Bcl-2比值显著降低(P<0.05)。与丁苯酞组比较,雷公藤甲素高剂量组cleaved Caspase-3、Bax相对表达量显著降低,Bcl-2相对表达量显著升高,Bax/Bcl-2比值显著降低(P<0.05)。见图4、表5。
组别 cleaved Caspase-3/
β-actinBcl-2/β-actin Bax/β-actin Bax/Bcl-2 假手术组 0.09±0.02 0.92±0.19 0.15±0.03 0.16±0.03 模型组 0.61±0.11* 0.18±0.04* 0.97±0.18* 5.39±1.07* 丁苯酞组 0.16±0.03△ 0.29±0.06△ 0.20±0.04△ 0.69±0.10△ 雷公藤甲素
低剂量组0.55±0.09 0.21±0.04 0.88±0.16 4.19±0.75△ 雷公藤甲素
中剂量组0.42±0.08△ 0.32±0.06△ 0.47±0.09△ 1.47±0.20△ 雷公藤甲素
高剂量组0.12±0.02△# 0.56±0.11△# 0.26±0.5△# 0.46±0.07△# *P<0.05,与假手术组比较;#P<0.05,与丁苯酞组比较;△P<0.05,与模型组比较。 -
线栓法是CIRI动物模型制备的经典方法,具有操作简便、重复率高、与人类临床病理接近等优点。本实验结果显示,CIRI模型大鼠呈现明显的神经功能障碍,BBB通透性异常升高,缺血半暗带大脑皮层神经元呈现形态不规则、胞体萎缩呈空泡样变、着色较深、核膜边界不清、炎性细胞浸润等病理学改变,与杨欢欢等[15]研究结果一致。本研究发现,经雷公藤甲素中、高剂量或丁苯酞预处理能够明显改善CIRI大鼠神经功能,降低脑梗死率和BBB通透性,改善缺血半暗带大脑皮层神经元病变并降低其凋亡率,并且雷公藤甲素高剂量组效果优于丁苯酞组;而雷公藤甲素低剂量组上述作用并不显著。说明雷公藤甲素具有抑制大鼠CIRI的作用,该作用具有一定的剂量依赖性。
CIRI病理机制非常复杂,其中炎症损伤和神经元凋亡发挥着关键作用。熊莉等[16]报道CIRI可诱导小胶质细胞活化而释放TNF-α、IL-1β等促炎因子,引发脑组织炎症反应发生。TNF-α和IL-1β具备炎性趋化属性,其中TNF-α可刺激促炎因子大量释放,IL-1β可诱导炎性细胞浸润,加重炎症反应[17]。Bcl-2和Bax是定位于线粒体膜的两种蛋白,二者均属于bcl蛋白家族,在细胞线粒体凋亡途径中发挥着重要作用。Bax可诱导细胞色素C由线粒体通过膜孔道进入细胞质,活化位于细胞质的Caspase-3,cleaved Caspase-3能够切割破坏膜蛋白、结构蛋白等引发细胞凋亡[18]。Bcl-2能够抑制Cyt C释放而表现为抑凋亡作用,并且Bcl-2与Bax能够结合形成无活性的二聚体。TLR4是定位于小胶质细胞的一种跨膜识别受体,TLR4能够诱导NF-κB磷酸化,p-NF-κB核转位后与DNA特定位点结合而诱导TNF-α、IL-1β等炎症因子转录与表达,进而加重炎症损伤[19]。此外,p-NF-κB可通过调控Bcl-2、Bax表达而诱导细胞凋亡[20]。Zhai Y等[21]发现抑制TLR4/NF-κB通路介导的炎症反应和凋亡可减轻大鼠CIRI。本实验结果显示,经雷公藤甲素中、高剂量或丁苯酞预处理能够明显降低CIRI大鼠缺血侧大脑皮层组织TNF-α、IL-1β含量和TLR4、cleaved Caspase-3蛋白相对表达量,降低p-NF-κB/NF-κB、Bax/Bcl-2比值,并且雷公藤甲素高剂量组效果优于丁苯酞组,而雷公藤甲素低剂量组上述作用并不显著。说明雷公藤甲素对CIRI大鼠炎症反应缺血半暗带大脑皮层神经元凋亡具有抑制作用,其机制可能与抑制TLR4/NF-κB通路活化有关,本结果与李晓蕾等[22]报道的药物抑制TLR4/NF-κB通路对局灶性脑缺血再灌注大鼠神经具有保护作用的结果相似。
综上所述,雷公藤甲素能够保护BBB通透性,减轻CIRI大鼠神经功能缺失和神经元病变,降低脑梗死率,作用机制可能与抑制TLR4/NF-κB通路及其介导的炎症反应和神经元凋亡有关。本研究结果为雷公藤甲素用于防治CIRI提供了理论依据。
Effects and mechanism of Triptolide on cerebral ischemia-reperfusion injury in rats
doi: 10.12206/j.issn.2097-2024.202311021
- Received Date: 2023-11-10
- Rev Recd Date: 2023-12-29
-
Key words:
- triptolide /
- cerebral ischemia-reperfusion /
- TLR4/NF-κB pathway /
- inflammation /
- apoptosis
Abstract:
| Citation: | ZHU Dongjie, HE Xinzheng, ZOU Jie, YU Shidan, LI Hongxia. Effects and mechanism of Triptolide on cerebral ischemia-reperfusion injury in rats[J]. Journal of Pharmaceutical Practice and Service. doi: 10.12206/j.issn.2097-2024.202311021 |









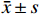


 DownLoad:
DownLoad: