-
肝癌(HCC)是常见的恶性肿瘤之一,尤其在我国特别高发,全球HCC患者超过半数在中国。2022年我国HCC发病率达36.77/10万人,位于恶性肿瘤第4位;总体病死率达31.65/10万人,居恶性肿瘤第2位[1]。HCC已然成为我国一项社会公共卫生问题,严重危害国民健康,给国民经济和社会发展带来沉重负担[2-4]。目前手术及肝移植可以临床治愈早期HCC,但多数患者诊断时已是晚期[5]。尽管免疫检查点抑制剂和小分子多靶点酪氨酸激酶抑制剂联合治疗法为晚期HCC患者带来了希望,但患者的中位生存期仅提高至19.2个月[6]。中医药治疗晚期HCC有其独特的优势,可以增强化疗、靶向、免疫治疗疗效,减轻毒副作用,改善症状,延长患者总生存期。因此运用中医药探索晚期HCC患者的治疗方案,对阻止该疾病的进一步发展,延长患者生存期具有重要的意义。
八宝丹起源于明代,至今有近400年的应用历史,是原国家食品药品监督管理总局允许使用天然麝香的特效药之一。八宝丹成分包含天然牛黄、天然麝香、蛇胆、羚羊角、珍珠、三七等。既往研究已证实,八宝丹可用于急慢性肝炎、胆囊炎等肝胆系统疾病,具有清热利湿、祛黄解毒的功效[7-8],并且八宝丹对HCC、胰腺癌、胆囊癌等消化系统肿瘤性疾病疾也有治疗效果[9-10]。本团队前期研究发现,八宝丹可以通过抑制慢性炎症介导的肝纤维化进而抑制HCC的发生,但八宝丹对于晚期HCC的治疗作用机制尚未明确[11]。因此探寻八宝丹对晚期HCC的作用机制可为中医药治疗HCC提供证据。
二乙基亚硝胺(DEN)诱导的大鼠模型是一个成熟的原发性HCC模型。DEN模型中HCC的发展过程与人类HCC相似,是一种非常合适研究HCC的生物工具[12]。本研究利用DEN大鼠观察八宝丹对HCC的影响,发现其能有效延缓HCC进展,但是作用机制尚不明确。由于八宝丹成分复杂多样,需要多组学分析技术探明八宝丹中有效药物成分与疾病的互作关系,因此本研究基于网络药理学和UPLC-MS方法,筛选八宝丹治疗HCC的有效化合物和潜在治疗靶点,构建“疾病-基因-药物”的多层次网络,揭示八宝丹调控疾病进展的潜在机制,为八宝丹的“老药新用”和HCC晚期患者的临床用药提供新思路。
-
八宝丹胶囊(批号Z10940006,厦门中药厂有限公司)、甲醇(A452-4,Fisher)、乙腈(A998-4,Fisher)、甲酸(A117-50,Fisher);DEN(245437, Sigma-Aldrich);高效液相色谱仪(ACQUITY UPLC I-Class HF,Water)、色谱柱(ACQUITY UPLC HSS T3,100 mm×2.1 mm, 1.8 μm, Water)、PDA检测器(ACQUITY Premier PDA eλ,Water);高分辨液质联用质谱仪(Thermo-Obritrap-QE,Thermo)。
-
将32只SD大鼠(6~8周龄,180~200 g,雄性,SPF级)随机分为 4 组,每组8只,分别为:对照组、八宝丹组、DEN 诱导组(DEN组)、DEN+八宝丹组。DEN组和DEN+八宝丹组连续饲喂12周DEN溶液(95 μg/ml), 对照组和八宝丹组饲喂灭菌水。八宝丹组不进行DEN诱导,从第12周开始予以八宝丹给药。DEN 诱导组,DEN饮水喂养大鼠持续12周,诱导HCC的发生,然后使用生理盐水灌胃,1次/2 d ,持续4周。DEN+八宝丹组,DEN诱导HCC发生后,八宝丹按照0.5 g/kg的剂量对实验动物进行灌胃,1次/2 d,持续4周(图1A)。实验结束后处死部分大鼠获取肝脏组织标本,剩余部分停药后继续观察生存期。
-
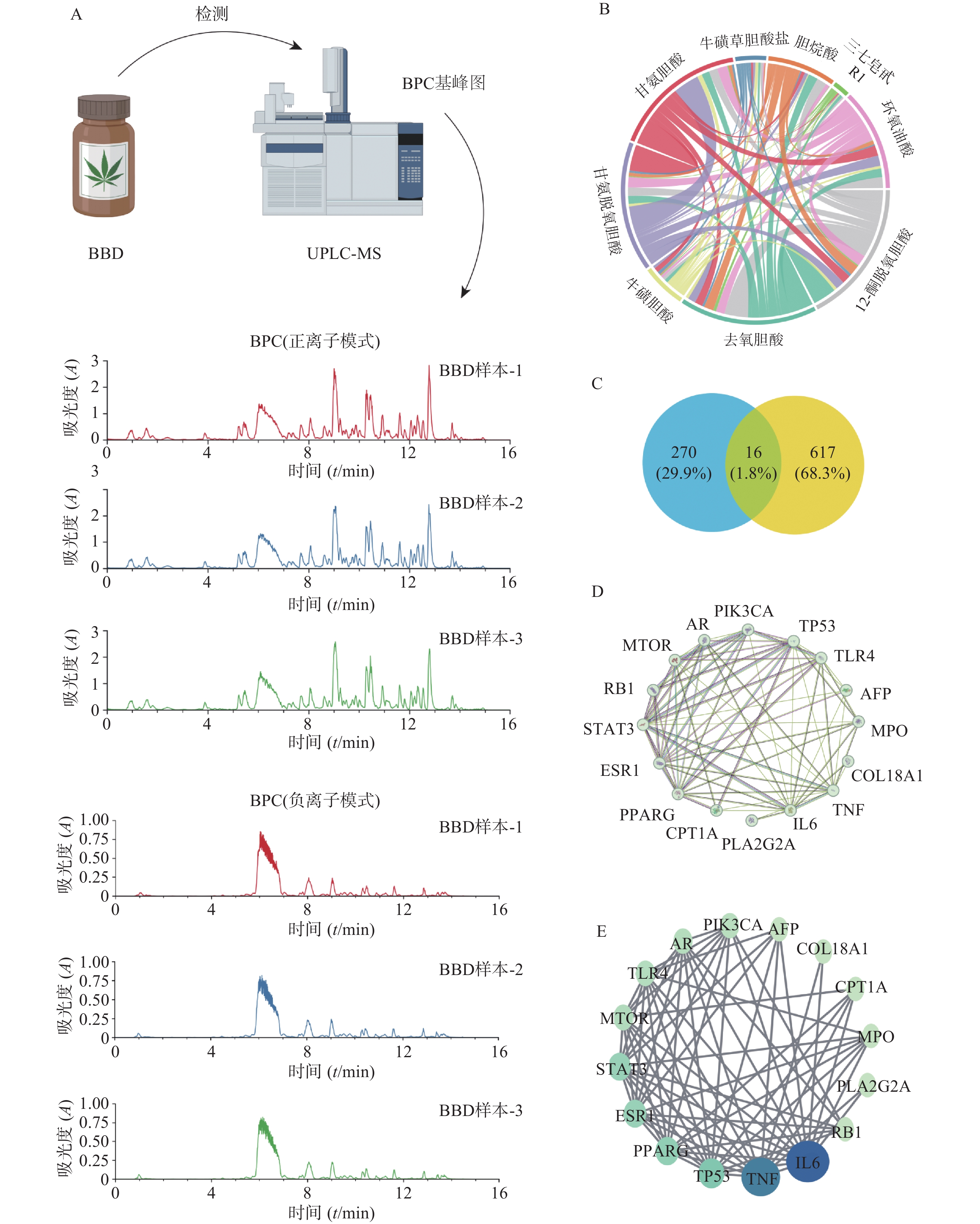
将八宝丹取出,用液氮研磨均匀后,称取约98.3 mg样本于1.5 ml离心管中;加入983 μl 甲醇-水溶液(V/V=3∶1,含混合内标,4 μg/ml),涡旋震荡1 min,加入钢珠;放入−40 ℃冰箱中预冷2 min后,在研磨机中研磨(60 Hz,2 min);冰水浴超声提取60 min,−40 ℃下静置30 min;离心10 min(12 000 r/min,4 ℃),取全部上清液过0.22 μm的有机相滤膜;4 ℃下静置过夜,离心10 min(12 000 r/min,4 ℃),取上清液过0.22 μm的有机相滤膜置于内衬管的LC-MS进样小瓶中进行分析。本次实验的分析仪器为ACQUITY UPLC I-Class HF超高效液相串联QE高分辨质谱仪组成的液质联用系统。色谱条件:色谱柱(ACQUITY UPLC HSS T3,100 mm×2.1 mm, 1.8 μm);柱温:45 ℃;流动相:水(含0.1%甲酸)和乙腈,梯度洗脱信息详见表1;流速:0.35 ml/min;进样体积:5 μl;PDA扫描范围210~400 nm。质谱条件:离子源(HESI,样品质谱信号采集分别采用正负离子扫描模式);数据采集模式为DDA;扫描方式为Full MS/dd-MS2(TOP 8);质谱参数详见表2。
时间(t/min) 水(含0.1%甲酸)(%) 乙腈(%) 0 95 5 2 95 5 4 70 30 8 50 50 10 20 80 14 0 100 15 0 100 15.1 95 5 16 95 5 参数 正离子 负离子 喷雾电压(U/V) 3 800 −3 000 毛细管温度(T/°C) 320 320 辅助气体加热器温度(T/ ℃) 350 350 鞘气流量(Arb) 35 35 辅助气体流速(Arb) 8 8 S透镜射频水平 50 50 质量范围(m/z) 100~1 200 100~1 200 全质谱分辨率 70 000 70 000 串联质谱分辨率 17 500 17 500 质谱仪二级碎裂能量 10,20,40 10,20,40 -
八宝丹 UPLC-MS数据由上海欧易生物医学科技有限公司提供,根据八宝丹化学成分在210 nm 和 254 nm波长下的紫外吸收图初步确定其结构类型。质谱数据经Progenesis QI v3.0 软件(Nonlinear Dynamics, Newcastle, 英国)处理(基线过滤、峰识别、积分、保留时间校正、峰对齐和归一化)获得八宝丹的质谱峰表。
-
八宝丹的质谱峰表中化合的鉴定基于精确质量数、二级碎片以及同位素分布,并结合TCM数据库(上海欧易生物医学科技有限公司,中国)进行鉴定。在本研究中,质谱峰表中对各鉴定物质进行评分,满分80分,其中,一级质谱精确分子量匹配,20分;二级质谱碎片匹配,20分;同位素分布匹配,20分;保留时间匹配,20分。将化合物相对峰面积的总含量定为100%,得到定性定量结果数据矩阵,并规定峰面积的比值大于1%,总分大于55,一级分子量误差在1.4×10−6以内的化合物作为八宝丹的有效成分进行后续分析。
-
根据前期筛选出的有效化学成分导入 PubChem(https://pubchem.ncbi.nlm.nih.gov/)数据库查找与有效活性成分相对应的Smiles号,并将Smiles号导入 Swiss Target Prediction(http://swisstargetprediction.ch/)和SuperPred(https://prediction.charite.de/subpages)数据库进行有效活性成分对应靶点的预测,保留结合可能性1%以上或文献报道过与化合物相关的作用靶点。根据各成分相关基因富集情况,利用R(version 3.6.1)构建各组分之间互作网络。
-
使用 GeneCards(https://www.genecards.org/)数据库、OMIM(https://omim.org/)数据库和Therapeutic Target Database(https://db.idrblab.net/)筛选HCC对应靶点,以“Hepatocellular carcinoma”为关键词进行筛选。并根据文献比对,保留与HCC相关的靶点用于后续研究。
-
根据前期有效成分相关靶点筛选和HCC相关靶点的选择,取交集进行后续分析。将共同靶点的数据导入STRING数据库(https://cn.string-db.org/)构建蛋白互作网络,再利用Cytoscape3.9.0软件,以介数中心数为参考,筛选出关键调控蛋白。
-
将有效成分和HCC之间的共有靶点导入 DAVID(https://david.ncifcrf.gov/)数据库,设置物种为人,P<0.01,分别勾选 GO 分析中的生物过程、细胞组成 、分子功能以及Pathway 中的 KEGG 进行富集分析,提取结果后,应用微生信(http://www.bioinformatics.com.cn/)平台将富集分析结果可视化。
-
将有效成分和HCC之间的共有靶点依次导入GEPIA2 (http://gepia2.cancer-pku.cn/)中,以182例TCGA数据库中保留的临床HCC患者的RNA测序数据为基础,分析共有靶点与患者生存期的相关性。
-
大鼠被处死后,用磷酸盐缓冲盐水和 4% 多聚甲醛进行经心灌注。取肝脏组织进行病理评估。石蜡包埋后肝脏切成 5 µm 厚的切片。染色时将HCC组织石蜡切片,60 ℃烘烤固片30 min;脱蜡至水,并按照生产商的方案用苏木精-伊红染色,最后镜下观察各组织病理学情况。
-
使用 GraphPad Prism 9.0 软件进行方差分析,每次实验的定量数据以平均值±标准差表示,组间差异采用t检验。Kaplan-Meier 分析用于进行生存期分析。采用对数秩检验比较各组存活时间。以P<0.05为差异有统计学意义。
-
在实验观察期间,对照组和八宝丹组的大鼠均存活,而DEN组平均生存期为17.1周,DEN+八宝丹组平均生存期为23周,是DEN组的1.35倍(图1B)。同时观察肝脏大体发现,对照组和八宝丹组肝脏组织未见异常;DEN 组肝脏组织中均出现大量肉眼可见肿瘤结节;而DEN+八宝丹组肝脏组织中虽然出现了部分肉眼可见的肿瘤结节,但大体形态上相较于DEN组损伤较轻(图1C)。通过对肿瘤结节的最大直径和数量测量统计发现DEN+八宝丹组肿瘤负荷和最大肿瘤直径显著小于DEN组(图1D)。同时,对各肝脏组织进行病理学评估和H&E染色结果发现,八宝丹组和正常肝脏组织组织排列紧密,未出现肝脏损伤,而DEN组出现明显的组织空泡化,组织间排列弥散,肝脏损伤严重。尽管DEN+八宝丹组大鼠肝脏组织相较于八宝丹组和正常对照大鼠出现了明显的炎症浸润和肝纤维化,但是,相较于DEN组的肝脏组织损伤较轻且组织排列较为紧密(图1E)。H&E染色结果表明,八宝丹对大鼠肝脏未发现实质性病理损伤,并且缓解了同期DEN大鼠的肝脏病理状态。上述结果表明,八宝丹能延缓DEN大鼠的生存期并减少肿瘤负荷,但是具体机制尚不明了。
-
为进一步探索八宝丹对HCC的影响机制,本研究首先对八宝丹进行UPLC-MS中药化学成分检测。中药成分鉴定流程如图2A所示,将八宝丹颗粒进行溶解、研磨、过滤和提纯后上机检测。紫外吸收图谱发现,210 nm处皂苷类成分在该波长下有强吸收,254 nm处含有苯环、双键等结构的成分在该波长下有强吸收。然后通过基峰色谱图反应样品的整体信息,3次重复性检测结果可知该检测方法离子相应响度、保留时间重复性良好,由此说明本次实验仪器稳定、数据可靠。原始数据经Progenesis QI软件处理和TCM数据库进行定性后,共发现851个中药成分,保留9个主要活性成分供后续研究,分别是牛磺草胆酸盐、12-酮脱氧胆酸、去氧胆酸、牛磺胆酸、甘氨脱氧胆酸、甘氨胆酸、三七皂苷R1、环氧油酸和3α,7α-二羟基-12-羰基-5β-胆烷酸。将主要活性成分信息分别导入PubChem和Swiss Target Prediction数据库,共获得285个潜在作用靶点,并绘制出化合物之间的互作网络,发现八宝丹中主要化学成分之间关系密切,相互存在多个共有靶点(图2B)。在Therapeutic Target Database、GeneCards、OMIM数据库和文献中共筛选出与HCC相关的靶点637个,保留八宝丹与HCC共有相关性靶点16个(图2C)作为潜在调控分子。将潜在调控分子导入STRING数据库构建蛋白与蛋白间互作网络(图2D),再利用Cytoscape3.9.0软件,以介数中心数(网络中所有最短路径中经过该节点的路径的数目占最短路径数的比例)为参考,将潜在调控分子进行排序,介数中心数越大,圆形的直径越大,颜色越蓝,说明在该网络中的关联度就越高,反之圆形的直径越小,颜色越绿(图2E)。其中,潜在调控分子介数中心数排名前5的分别是:白介素-6(IL-6)、肿瘤坏死因子(TNF)、细胞肿瘤抗原p53(TP53)、过氧化酶增殖因子活化受体-γ(PPARG)和雌激素受体1(ESR1)。
-
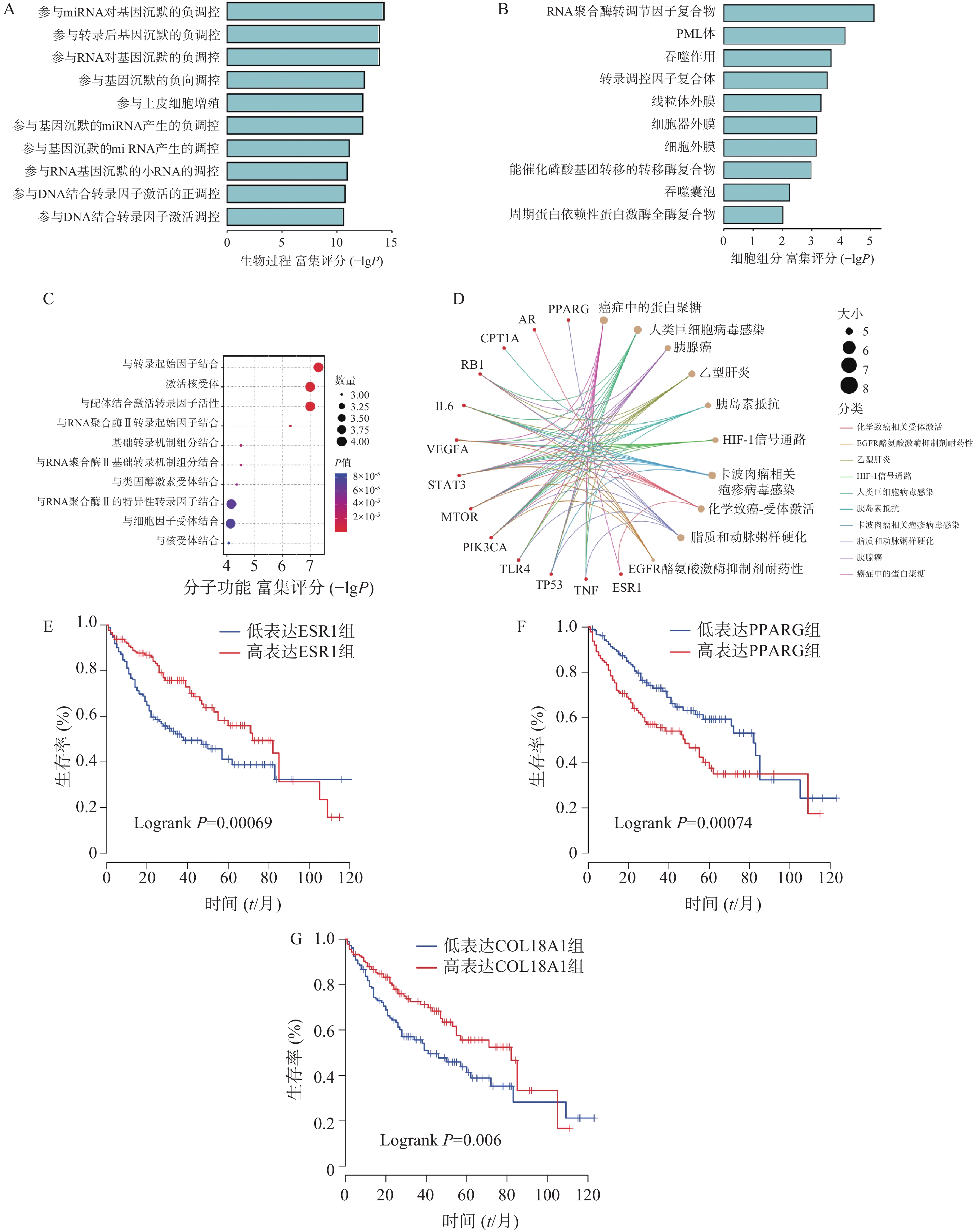
为探索八宝丹调控HCC的潜在作用机制,本研究基于DAVID数据库将上述16个共有相关靶点进行GO及KEGG富集分析。GO富集分析显示802个生物过程、11个细胞组成、43个分子功能,KEGG通路富集分析共90条通路,展示排名前10的富集条目。
图3A提示八宝丹在生物过程中通过沉默细胞中mRNA的转录,抑制上皮样细胞的增殖和转录因子的结合调控HCC;图3B提示八宝丹有效化学成分在细胞层面上通过结合各种细胞器或细胞膜受体发挥作用;图3C提示八宝丹有效化学成分能调控转录因子的结合、细胞因子的分泌及细胞核的状态。图3D提示八宝丹调控HCC是通过多通路、多靶点之间的相互作用。最后在TCGA数据库中查询了共有相关靶点与HCC患者的临床相关性,在182例临床样本的调查中发现,3个基因的高表达提示着患者的总生存期较短,分别为ESR1 (Logrank P=0.000 69)、PPARG(Logrank P=0.000 74)和COL18A1(Logrank P=0.006),详见图3E~G,P值由Kaplan-Meier生存曲线和log-rank检验确定。
-
HCC是一种典型的炎症相关癌症,近90%的HCC与病毒性肝炎、过度酒精摄入、非酒精性脂肪肝或酒精性脂肪肝引起的长期炎症相关。在HCC慢性炎症微环境中,先天免疫细胞和成纤维细胞被募集并激活至损伤部位分泌细胞因子和生长因子,有利于肿瘤细胞的增殖或抵消凋亡[6]。研究表明,NF-κB和JAK-STAT两条经典通路是促进HCC的关键炎症信号通路,抑制炎症通路的激活能有效抑制住HCC进一步进展[13]。八宝丹作为传统中药复方,临床上已发现对HCC、胰腺癌、胆囊癌等消化系统肿瘤性疾病疾也有着积极的治疗效果,但是具体机制尚不明了。本团队前期研究发现,八宝丹可以通过抑制炎症细胞因子分泌和减少肝前体细胞上TRL4的表达,从抑制肝前体细胞的肿瘤转化,进而抑制肿瘤发生[11]。因此本研究团队假设八宝丹减缓了原发性HCC的肿瘤进展。
在本研究中,通过观察八宝丹对DEN诱癌大鼠的影响,发现八宝丹可以延长DEN大鼠的生存期,并且缓解DEN导致的肝脏肿瘤负荷。但是,目前尚未有具体的机制阐明这一现象。为探索是八宝丹中何种中药成分发挥了作用,利用UPLC-MS技术检测出化学成分851个,鉴定出主要活性成分9个,其中大部分是人体胆汁酸中主要成分,也发现具有强抗炎作用的三七皂苷R1[14],主要活性成分之间关系密切。共筛选出285个潜在作用靶点。在与HCC相关靶点取交集后,筛选出八宝丹调控HCC的16个潜在靶点。利用上述靶点构建了八宝丹“化学成分-潜在靶点”网络模型以及PPI网络,将潜在作用靶点进行排序,发现了HCC小鼠模型已验证能加速HCC的发展并影响肿瘤的侵袭性免疫信号IL-6和TNF[15];HCC中的显性突变驱动因子TP53和RB1[16];与HCC炎症相关的信号分子STAT3;与肿瘤细胞增殖相关的分子MTOR和AFP[17]。
GO富集分析结果提示,八宝丹调控HCC在生物过程上参与miRNA对基因的沉默负调控、转录后miRNA对基因的沉默负调控、RNA对基因的沉默负调控等;在细胞组分上富集到RNA聚合酶Ⅱ转录调节因子复合物、吞噬作用相关受体、细胞膜受体等词条;在分子功能上富集到与转录起始因子结合,激活核受体,与配体结合激活转录因子活性等。KEGG信号通路提示,八宝丹参与了癌症中的蛋白聚糖、乙型肝炎、HIF-1信号通路、化学致癌受体激活、EGFR络氨酸激酶抑制剂耐药等通路。TCGA数据库中发现,ESR1、PPARG和COL18A1高表达与HCC患者总生存期密切相关。据报道,PPARG高表达可激活WNT/CTNNB1信号通路,从而维持肿瘤细胞干性特征,促进癌症进展[18]。人类HCC临床样本调查和组织染色也证实,ESR1可通调控miR-141-3p /GSN信号影响原发性HCC进展[19];肌成纤维细胞分泌的胶原α-1(ⅩⅤⅢ)链(COL18A1)可促进肿瘤转移,加速HCC进展[20]。因此TCGA结果提示,八宝丹可能是通过调控ESR1、PPARG和COL18A1来延长原发性HCC患者的生存期。
综上所述,本研究证实了八宝丹对治疗原发性HCC的积极影响,并且发现9种八宝丹的有效活性成分。还利用网络药理学的研究方法,分析揭示八宝丹可能是通过多靶点、多通路来调控原发HCC,并结合已有的研究进行分析验证,提出新的视角与思路,为后续的实验研究提供参考。
Potential mechanism of Babao Dan in the treatment of hepatocellular carcinoma based on network pharmacology
doi: 10.12206/j.issn.2097-2024.202401061
- Received Date: 2024-01-26
- Rev Recd Date: 2024-03-18
- Available Online: 2024-04-24
- Publish Date: 2024-04-25
-
Key words:
- hepatocellular carcinoma /
- Babao Dan /
- network pharmacology /
- UPLC-MS /
- mechanism of action
Abstract:
| Citation: | ZHU Xinyu, BAI Haoran, ZHAO Naping, QI Dachuan, WEI Lixin, ZHANG Li. Potential mechanism of Babao Dan in the treatment of hepatocellular carcinoma based on network pharmacology[J]. Journal of Pharmaceutical Practice and Service, 2024, 42(4): 157-164. doi: 10.12206/j.issn.2097-2024.202401061 |









 DownLoad:
DownLoad: