-
紫杉醇(PTX)是从裸子植物红豆杉树皮分离得到的天然次生代谢产物,属于微管蛋白抑制剂,在临床上广泛用于卵巢癌、非小细胞肺癌和乳腺癌的治疗[1]。传统的PTX注射液因添加了聚氧乙烯蓖麻油而导致过敏反应,并加重PTX的外周神经毒性,还影响药物分子向组织间扩散,影响抗肿瘤效应,且滴注时间过长[2]。前药设计在提高药物生物利用度,增加药物稳定性,减小毒副作用,延长药物作用时间等方面具有很大的优势[3]。其中,长链饱和脂肪酸如肉豆蔻酸(MA)[4]、棕榈酸(PA)[5]和硬脂酸(SA)[6]等常用于药物成酯前药修饰。例如,治疗类风湿性关节炎的上市药物地塞米松棕榈酸酯注射液,就是通过饱和脂肪酸棕榈酸与地塞米松成酯反应形成地塞米松棕榈酸酯前药,继而制备成纳米前药,极大地增加了制剂的稳定性与成药性[6]。
脂质体作为载体递送脂溶性药物近年来受到广泛关注,这为PTX脂质体制剂的研发提供了重要依据。据此,我们对PTX的C2′羟基进行酯化修饰形成脂溶性较强的PTX肉豆蔻酸酯(PTX-MA)、PTX棕榈酸酯(PTX-PA)和PTX硬脂酸酯(PTX-SA)等前药分子,然后将其分别制备成PTX-MA脂质体(PTX-MA-L)、PTX-PA脂质体(PTX-PA-L)和PTX-SA脂质体(PTX-SA-L),不仅可以解决PTX成药性差的问题,也可以提高载药量、增加药物稳定性、改善药代动力学和药效学[7-10]。
该研究以卡马西平为内标,成功建立了小鼠血浆中PTX-MA、PTX-PA和PTX-SA含量的超高效液相色谱-串联质谱(UPLC-MS/MS)测定方法,在此基础上比较考察了其脂质体PTX-MA-L、PTX-PA-L和PTX-SA-L在小鼠体内的药代动力学特性,从而为PTX脂肪酸酯前药的纳米制剂研发提供科学依据。
-
Vanquish™ 超高相液相色谱仪(美国Thermo scientific公司);TSQ Altis™ 三重四级杆质谱仪(美国Thermo scientific公司);台式高速冷冻离心机(上海伟进生物科技有限公司)。
-
紫杉醇(纯度≥98%)购自江苏红豆杉生物医药科技股份有限公司;紫杉醇肉豆蔻酸酯(PTX-MA,纯度≥98%)及其脂质体(PTX-MA-L)、紫杉醇棕榈酸酯(PTX-PA,纯度≥98%)及其脂质体(PTX-PA-L)、紫杉醇硬脂酸酯(PTX-SA,纯度≥98%)及其脂质体(PTX-SA-L)均为海军军医大学药学系药剂学教研室自制;甲酸、超纯水、甲醇和乙腈购自赛默飞世尔(中国)有限公司,均为色谱纯;卡马西平购自上海源叶生物科技有限公司。
-
ICR小鼠[雌性,(18±2) g,购自浙江维通利华实验动物技术有限公司,动物许可证号:SCXK(浙)2020-0002]。
-
色谱柱:Eclipse Plus C8色谱柱(2.1 mm×50 mm,1.8 μm);流动相:0.2%甲酸水溶液(A)-甲醇(B)梯度洗脱;梯度洗脱程序:20% B~ 60% B(0~0.3 min),60% B~ 98% B(0.3~ 3.9 min),98% B~ 60% B(3.9~ 4.8 min),60% B~ 20% B(4.8~ 5.2 min),20% B(5.2~ 7.0 min);流速:0.3 ml/min;进样量:10 µl;柱温:30 ℃。
-
电喷雾离子源(ESI)采用正离子采集模式。离子源参数:电喷雾电压:3.5 kV(+);离子传输管温度:325 ℃;雾化温度:275 ℃;鞘气压力:35 Arb;辅气压力:5 Arb;毛细管压力:1.5 mTorr。定量分析离子分别为:m/z
1086.7 →518.3(PTX-MA),m/z1114.5 →546.3(PTX-PA),m/z1142.7 →574.4(PTX-SA),m/z 237.1→194.0(卡马西平,内标)。 -
分别精密称取10.00 mg 3种对照品(PTX-MA、PTX-PA、PTX-SA)置于10 ml容量瓶中,用甲醇溶解并定容,配制成1.00 mg/ml的对照品储备液。吸取一定量的对照品储备液,用甲醇稀释成一系列浓度的工作溶液,置于4 ℃冰箱保存备用。
-
精密称取10.00 mg卡马西平,用乙腈溶解并定容,配制成1.00 mg/ml的内标储备液。吸取一定量的内标储备液,用乙腈稀释成30.00 ng/ml工作溶液,置于4℃冰箱保存备用。
-
精密量取100 μl小鼠血浆样品液于1.5 ml离心管中,加入300 µl内标溶液,涡旋混合1 min后,13 000 r/min离心10 min,吸取上清液,置于新的EP管中,于40 ℃下真空离心挥干溶剂。精密量取200 µl乙腈复溶样品,过0.22 μm尼龙滤膜,即制得小鼠血浆样品溶液。
-
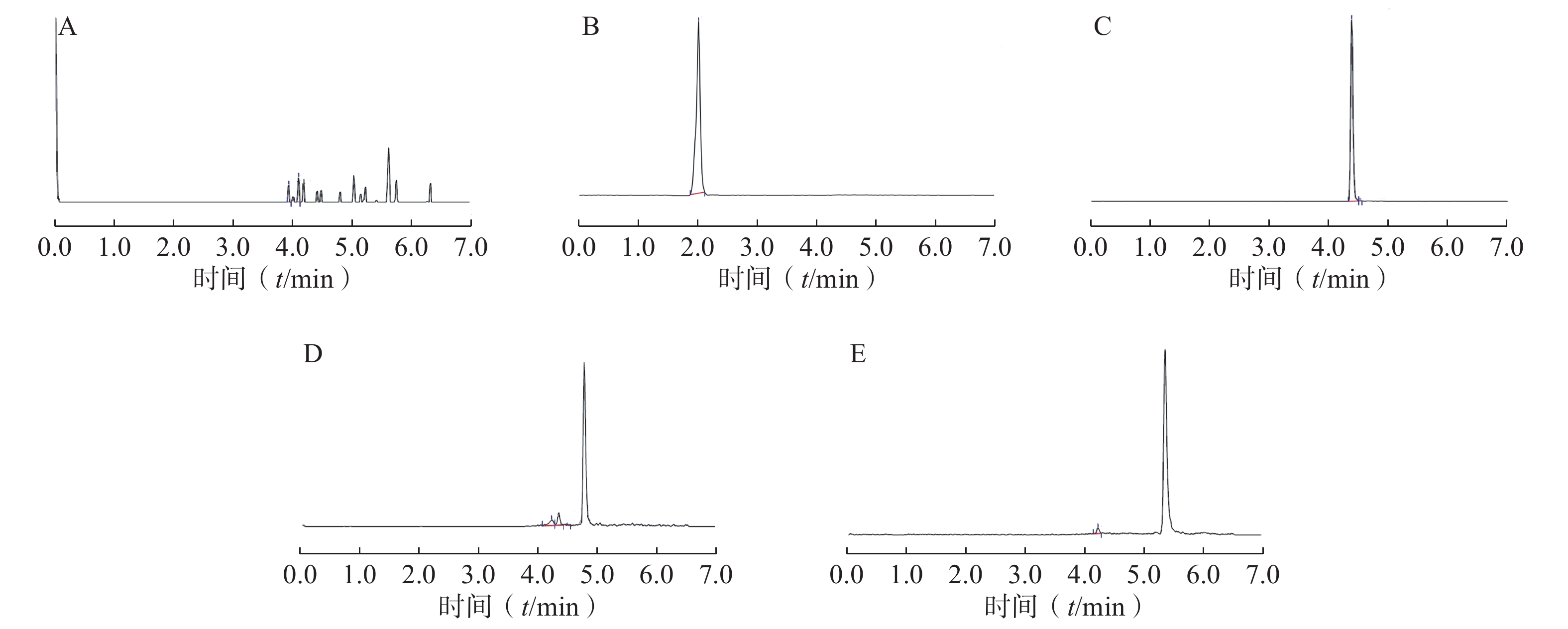
精密量取100 µl空白血浆,用300 µl乙腈代替300 µl内标液,按“2.3”项下方法处理后,再按“2.1”项下方法测定,记录空白血浆色谱图;精密量取100 µl空白血浆,按“2.3”项下操作处理后,再按“2.1”项下方法测定,记录含内标的血浆样品色谱图;精密量取含适量PTX-MA、PTX-PA、PTX-SA的空白血浆样品各100 µl,用300 µl乙腈代替300 µl内标液,按“2.3”项下方法处理后,再按“2.1”项下方法测定,记录含PTX-MA、PTX-PA和PTX-SA的血浆样品色谱图。结果见图1,内标、PTX-MA、PTX-PA和PTX-SA的色谱峰附近无明显内源性杂质峰干扰,其保留时间分别为2.41、4.37、4.84和5.28 min,表明血浆中内源性物质不干扰PTX-MA、PTX-PA、PTX-SA和内标的测定。
-
精密量取95 µl小鼠空白血浆,加入5 µl紫杉醇脂肪酸酯对照样品(PTX-MA或PTX-PA或PTX-SA)工作溶液配制成5.00、25.00、50.00、100.00、300.00和500.00 ng/ml的PTX-MA或PTX-PA或PTX-SA系列浓度对照样品小鼠血浆液,按“2.3”项下操作处理后,再按“2.1”项下进样测定。以PTX-MA或PTX-PA或PTX-SA对照样品浓度为横坐标(X),样品峰面积与内标峰面积的比值为纵坐标(Y)进行线性回归分析,求得各自回归方程,结果见表1。
对照样品 回归方程 r 线性范围(ng/ml) PTX-MA Y=0.186 2 X−1.984 0.995 8 5.00~500.00 PTX-PA Y=0.668 5 X−12.977 0.998 4 5.00~500.00 PTX-SA Y=0.402 1 X−7.171 0.998 8 5.00~500.00 -
取空白小鼠血浆,分别配制含PTX-MA、PTX-PA和PTX-SA浓度为10.00、250.00和375.00 ng/ml的低、中、高3个浓度的小鼠血浆样品溶液,置于−80 ℃低温冰箱保存。按“2.3”项下方法处理后,再按“2.1”项下方法进样分析测定。每个浓度5份,每天连续进样3次,连续3 d。根据测得PTX-MA、PTX-PA、PTX-SA和内标的峰面积,计算PTX-MA、PTX-PA和PTX-SA的实测浓度,考察PTX-MA、PTX-PA和PTX-SA低、中、高浓度在小鼠血浆中的日内及日间的准确度和精密度。如表2结果显示,PTX-MA、PTX-PA和PTX-SA在低、中、高3个浓度的日内和日间的精密度RSD均<8.4%,准确度均>95%,表明仪器精密度良好。
对照样品 理论浓度(ng/ml) 日内精密度 日间精密度 实测浓度(ng/ml) 回收率(%) RSD(%) 实测浓度(ng/ml) 回收率(%) RSD(%) PTX-MA 10.00 9.93±0.83 99.36±8.32 8.38 10.37±0.61 103.67±6.06 5.84 250.00 242.29±10.93 96.84±4.51 4.51 247.42±5.30 98.97±2.12 2.14 375.00 371.31±6.30 99.02±1.68 1.70 373.37±6.20 99.57±1.65 1.66 PTX-PA 10.00 9.83±0.50 98.28±5.00 5.09 10.25±0.55 102.53±5.52 5.38 250.00 248.42±6.81 99.37±2.72 2.74 246.93±5.74 98.77±2.29 2.32 375.00 373.79±8.70 99.68±2.32 2.33 371.59±4.12 99.09±1.10 1.11 PTX-SA 10.00 9.59±0.51 95.90±5.08 5.30 10.33±0.67 103.27±6.72 6.50 250.00 248.81±7.89 99.53±3.16 3.17 245.92±5.04 98.37±2.01 2.05 375.00 374.36±8.81 99.83±2.35 2.35 371.26±2.43 99.00±0.65 0.65 -
取空白小鼠血浆,分别配制低、中、高3个浓度(10.00、250.00和375.00 ng/ml)的血浆样品(PTX-MA、PTX-PA和PTX-SA)液各3份,分别于室温放置12 h、−20 ℃冻存5 d后复融或−20 ℃反复冻融3次,按“2.3”项下方法处理样本,再按“2.1”项下方法进样分析测定。结果显示,在上述考察条件下,PTX-MA、PTX-PA和PTX-SA的低、中、高3个实测浓度的RSD均<10%,表明PTX-MA、PTX-PA和PTX-SA的小鼠血浆样品在室温放置12 h、−20 ℃冻存5 d及−20 ℃反复冻融3次条件下稳定性良好。
-
取空白小鼠血浆,分别配制低、中、高3个浓度(10.00、250.00和375.00 ng/ml)的血浆样品(PTX-MA、PTX-PA和PTX-SA)液各3份,按“2.3”项下方法处理样本,再按“2.1”项下方法进样分析测定,计算所得浓度为A。另取空白血浆,按“2.3”项下方法处理样本后,再加入PTX-MA或PTX-PA或PTX-SA工作液形成终浓度分别为10.00、250.00和375.00 ng/ml的3种不同浓度的标准溶液,按“2.1”项下方法进样分析测定,计算所得浓度为B。用甲醇配制含内标(30 ng/ml)的低、中、高3个浓度(10.00、250.00 和375.00 ng/ml)的PTX-MA或PTX-PA或PTX-SA的样品溶液,按“2.1”项下方法进样分析测定,计算所得浓度为C。提取回收率为(A/B)×100%,基质效应为(B/C)×100%。结果见表3,PTX-MA、PTX-PA或PTX-SA的平均提取回收率和基质效应的RSD均<10%,符合测定要求。
对照样品 理论浓度(ng/ml) 回收率(%) RSD(%)a 基质效应(%) RSD(%)b PTX-MA 10.00 79.82±6.60 8.28 95.36±6.55 6.87 250.00 88.35±2.84 3.22 96.28±3.59 3.73 375.00 92.08±2.46 2.67 98.86±1.46 1.48 PTX-PA 10.00 73.34±7.24 9.88 94.28±1.96 2.08 250.00 83.58±2.24 2.68 98.46±1.86 1.89 375.00 89.47±1.51 1.68 98.88±0.68 0.69 PTX-SA 10.00 61.90±4.11 6.63 95.90±5.08 5.30 250.00 77.26±2.88 3.73 98.78±2.22 2.25 375.00 84.43±1.21 1.44 99.08±1.01 1.02 注:a:回收率的相对标准偏差;b:基质效应的相对标准偏差。 -
雌性ICR小鼠90只,于实验前12 h禁食不禁水, 标记称重后分为PTX-MA-L、PTX-PA-L、PTX-SA-L 3组。以紫杉醇等摩尔剂量15.00 mg/kg小鼠尾静脉给药,分别于给药后2 min、30 min、1 h、2 h、4 h、6 h、12 h、1 d、3 d、14 d,小鼠眼眶静脉丛取血0.3 ml(每个时间点各取3只小鼠),置于装有肝素钠的离心管中,以10 000 r/min离心3 min后,上清液置于−80 ℃冰箱保存待测。上述血浆样品液按“2.3”项下方法处理后按“2.1”项下方法进样测定。所得数据用DSA2.0软件进行处理,以三房室模型计算得到药代动力学参数,结果见表4。
关键参数 单位 PTX-MA-L PTX-PA-L PTX-SA-L Cmax ng/L 226 436.10±4 932.89 289 171.80±5 311.62 333 508.00±3 464.10 AUC0-14 d ng·h/L 502 384.75±3 464.10 776 973.44±5 196.15 1 668 984.05±6 350.85 AUC0-∞ ng·h/L 503 800.86±8 082.90 777 835.54±6 429.10 1 669 696.54±5 773.50 t1/2 h 14.78±2.00 44.49±3.51 69.32±2.15 V L/kg 45.68±1.00 62.57±1.53 68.58±3.10 CL L·kg/h 29.06±2.52 24.94±2.08 13.74±2.52 -
该研究以卡马西平为内标,建立了小鼠血浆中3种紫杉醇脂肪酸酯前药含量的UPLC-MS/MS测定方法。其中,内标、PTX-MA、PTX-PA和PTX-SA色谱峰保留时间分别为2.41、4.37、4.84和5.28 min,均达到基线分离。相比前期研究中血浆PTX-MA、PTX-PA和PTX-SA含量的传统HPLC测定方法存在分离度差、灵敏度低的缺陷而无法满足检测要求,该方法具有选择性更好、分离范围更广、分离能力更强、灵敏度及重现性更高等优点,并成功应用于3种紫杉醇脂肪酸酯前药脂质体(PTX-MA-L、PTX-PA-L和PTX-SA-L)的小鼠体内药代动力学参数的测定。
该研究采用乙腈直接沉淀血浆蛋白的方式,短时间内可检测小鼠血浆中3种紫杉醇脂肪酸酯,体现了方法的便捷性和可控性。另外,通过优化流动相组成以及比例,使得内源性杂质与药物样品具有较好的分离效果。特别是在流动相中加入甲酸,不仅可以改善峰形,也可促进样品离子化,提高质子化检测能力,从而提高该方法的检测灵敏度。
总之,该研究建立的UPLC-MS/MS测定方法专属性强、操作简便、灵敏度和精密度高、重现性和稳定性好, 可用于定量测定小鼠体内血浆中3种紫杉醇脂肪酸酯前药含量。小鼠体内药代动力学研究结果显示, 3种紫杉醇脂肪酸酯脂质体PTX-MA-L、PTX-PA-L和PTX-SA-L在小鼠体内的t1/2分别为14.78、44.49和69.32 h,清除率(CL)分别为29.06、24.94和13.74 L·kg/h,提示随着脂肪酸碳链长度的增加,紫杉醇脂肪酸酯在小鼠体内的t1/2大幅延长、CL则显著降低,表明紫杉醇经不同链长饱和脂肪酸酯化修饰可明显改变其体内药动学特性,其中,紫杉醇棕榈酸酯脂质体(PTX-PA)具有适宜的药动学参数,值得下一步深入开发研究。
Determination and pharmacokinetics investigation of prodrugs of paclitaxel fatty acid esters in mouse plasma by UPLC-MS/MS
doi: 10.12206/j.issn.2097-2024.202404082
- Received Date: 2024-04-23
- Rev Recd Date: 2024-06-18
- Available Online: 2024-08-22
- Publish Date: 2024-08-25
-
Key words:
- Paclitaxel /
- Fatty acid esters /
- Prodrug /
- UPLC-MS/MS /
- Pharmacokinetics
Abstract:
| Citation: | CHEN Bingchen, TONG Dafeng, WAN Miao, YAN Feihu, YAO Jianzhong. Determination and pharmacokinetics investigation of prodrugs of paclitaxel fatty acid esters in mouse plasma by UPLC-MS/MS[J]. Journal of Pharmaceutical Practice and Service, 2024, 42(8): 341-345. doi: 10.12206/j.issn.2097-2024.202404082 |








 DownLoad:
DownLoad: