-
顺式阿曲库铵(cisatracurium, CA)是一种新型中时效非去极化肌松药,其结构式如图1,因其代谢方式(Hofmann elimination,霍夫曼降解)独特,同时释放较低水平的组胺,在临床麻醉过程中被广泛应用。因与其他非去极化肌松药一样,存在术后肌松残留作用[1],且已成为麻醉后呼吸系统不良事件的主要原因。因此,为保证患者围术期安全,对顺式阿曲库铵进行血药浓度监测十分必要。
CA的稳定性主要受温度和pH影响,温度和pH升高可加快其消除,因此离体后的生物样本稳定性是血药浓度监测方法建立的一个难点。国内外文献报道测定人血浆中CA浓度的方法主要为高效液相色谱荧光检测法(HPLC-FD)及高效液相色谱串联质谱法(LC-MS)[2-6],但样本前处理方法较为复杂。本研究在既往文献报道的基础上,建立了一种更加简便、准确、快速、稳定的LC-MS方法,适用于人血浆中CA的血药浓度测定。
HTML
-
高效液相色谱仪(Agilent 6410,美国Agilent公司);Triple Quad三重四极杆质谱仪,配电喷雾(ESI)离子源(美国Agilent公司);纯水机(Millipore);分析天平(BP211D,德国Sartorius公司)。对照品顺式阿曲库铵(cisatracurium,CA,批号:C496700,纯度90%,Toronto Reasearch Chemicals Inc公司);对照品盐酸普罗帕酮(propafenone hydrochloride,PPF,批号:101190-201101,纯度99.9%,中国药品食品检定研究院);乙腈、甲酸均为质谱纯(美国TEDIA公司);水为自制超纯水。
-
Agilent SB-C18色谱柱(2.1 mm×50 mm, 3 µm),柱温35 ℃;流动相A为0.1%甲酸水溶液,B为0.1%甲酸乙腈溶液。线性梯度洗脱:0 ~1 min, 20% B;1~3 min,50% B;3 ~5 min,20% B; 5 ~10 min,20% B。流速0.3 ml/min;进样量2 µl。
-
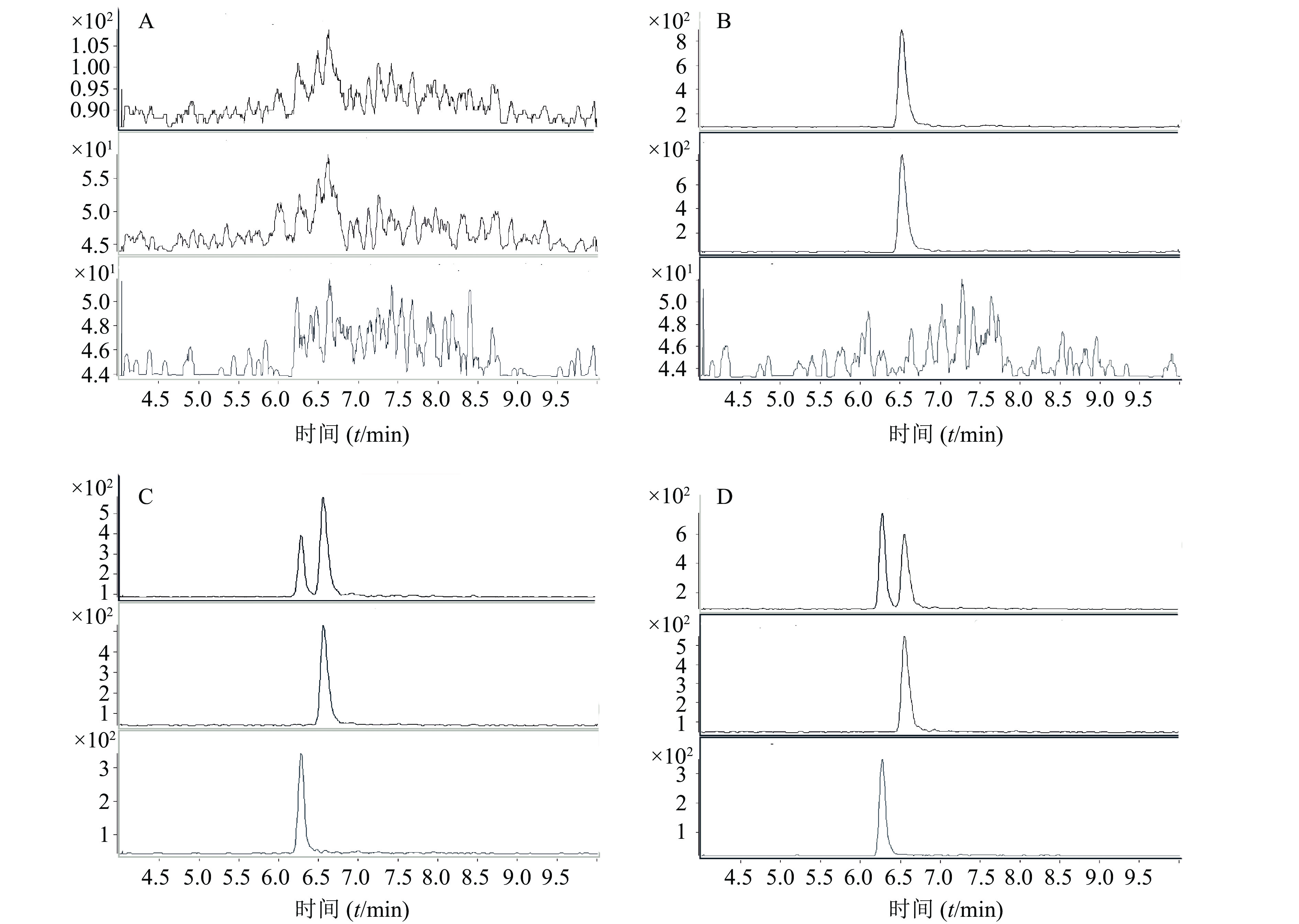
采用正离子工作模式;离子源为ESI源;多反应监测(multiple reaction monitoring, MRM)。毛细管电压4 000 V;干燥器温度350 ℃;干燥气流速12 L/min;雾化器压力35 psi。MRM监测离子对,离子驻留时间、碰撞诱导解离电压及碰撞能量等参数见表1。典型质谱图见图2。
成分 母离子 子离子 驻留时间(t/ms) 碰撞电压(U/V) 电子倍增电压( U/V) 碰撞能量(U/V) CA 464.4 358.4 20 110 200 20 PPF 342.2 166.2 200 130 200 17 -
CA标准储备液:精密称取CA 10 mg置10 ml容量瓶,加入2%甲酸甲醇溶液至刻度,溶解混匀,配制成相当于CA质量浓度为1 mg/ml的储备液,于冰箱(4 ℃)备用。
CA标准工作液:精密吸取CA储备液适量,用50%甲醇水溶液配制成系列浓度的工作溶液,其中,CA质量浓度分别为5 000、2 500、1 000、500、250、100、20 ng/ml。
PPF标准储备液:精密称取PPF 0.4 mg置2 ml容量瓶,加甲醇至刻度,溶解混匀,配制成相当于PPF质量浓度为0.2 mg/ml的储备液,于冰箱(4 ℃)备用。
PPF标准工作液:精密吸取PPF储备液适量,配制成200 ng/ml的含内标乙腈溶液。
-
精密吸取血浆30 μl,加入2%甲酸水溶液30 μl,振摇10 s,再加50 μl含内标乙腈溶液后旋涡振荡10 s,12 000 r/ min离心10 min,转移上清液置进样瓶,进样。
-
取空白人血浆,精密加入不同浓度的对照品溶液,配制成CA浓度为500、250、100、50、25、10、2 ng/ml的血浆标准品。以浓度为X轴,样品峰与内标峰的比值为Y轴,进行线性回归,经“1/X”权重得回归方程分别为:Y = 7.43×10–3 + 1.99 × 10–2X (r = 0.996 5)。结果表明,CA在2~500 ng/ml内,线性关系良好。本方法CA的定量下限为2 ng/ml。
-
配制CA浓度分别为8、80、400 ng/ml的血浆样品,日内误差以3个浓度各5个样品的测定结果表示;日间误差以3个浓度样品分别3个批次结果表示。人空白血浆分别加入不同浓度CA工作溶液,按照“2.3”项下方法提取后,相同色谱条件进行浓度测定。同时测定用流动相稀释为相应浓度的无基质溶液的峰面积,以计算绝对回收率。结果如表2所示。
CA理论浓度(ng/ml) 日内 日间 测得浓度(ng/ml) 精密度RSD(%) 准确度REC(%) 测得浓度(ng/ml) 精密度RSD(%) 准确度REC(%) 8 8.53 2.39 106.67 8.52 2.00 106.46 80 89.54 1.77 111.93 88.16 3.48 110.19 400 390.53 15.9 97.63 398.59 5.19 99.65 -
空白血浆分别配制成8、80、400 ng/ml的样品,每批次各3个,分别考察其①室温下放置4 h;②处理后室温放置24 h;③-80 ℃放置14 d的稳定性。结果如表3所示。
时间 样品浓度(ng/ml) REC(%) RSD(%) 室温4 h 低 103.50 9.28 中 98.27 5.26 高 101.33 3.73 处理后室温24 h 低 77.22 5.37 中 112.95 10.83 高 110.46 6.80 –80 ℃ 14 d 低 75.40 7.95 中 113.67 23.61 高 98.22 1.69 -
取6个不同来源的人空白血浆30 μl,除不加内标外,其余按“2.3”项下操作,取上清液,分别制备成CA浓度为31.25和1 000 ng/ml;PPF浓度为200 ng/ml的溶液,进样测定,记录峰面积。另用流动相将CA稀释成31.25和1 000 ng/ml;PPF浓度为200 ng/ml浓度的溶液,进样测定,记录峰面积。
基质效应=(空白基质中添加工作溶液峰面积/重组溶液中添加工作溶液峰面积)×100%
CA的基质效应为52%(RSD 2.09%~15.33%)。PPF的基质效应为96.71%(RSD4.6%)。
-
采用肌松仪监测患者注射苯磺顺式阿曲库铵后神经肌肉阻滞程度。给予4个成串刺激(train of four stimulation,TOF),给药后,T4最先发生衰减,T1最后衰减。当T1消失即仪器显示为0时,肌肉阻滞程度最大,随后逐渐恢复。分别在T0(T1消失至0)、TOFr 50(恢复至50%)、TOFr 90(恢复至90%)时采集输液对侧动脉血3 ml,并及时保存于4 ℃冰箱中。采用建立的方法测定样本血药浓度,结果见表4。同时记录T0(给药结束到T1消失至0)、TOFr50(给药结束到恢复至50%)、TOFr90(给药结束到恢复至90%)发生所需时间,结果见表5、表6。
TOF (%) 浓度(ng/ml) 受试者1 受试者2 受试者3 受试者4 受试者5 受试者6 0 445.02 525.30 253.83 165.29 150.57 309.22 50 76.58 125.65 68.71 68.99 53.18 58.46 90 44.10 71.80 60.72 71.74 41.12 45.07 基本信息 受试者1 受试者2 受试者3 受试者4 受试者5 受试者6 性别 女 女 女 男 女 男 年龄(岁) 50 49 53 70 36 40 体重(m/kg) 70 75 60 50 53 80 身高(l/cm) 165 165 164 161 165 173 TOF(%) 时间(t/min) 0 10 15 15 20 20 20 50 70 70 65 65 65 75 90 105 95 95 100 85 110
2.1. 分析条件
2.1.1. 色谱条件
2.1.2. 质谱条件
2.2. 对照品及内标溶液配制
2.3. 血浆样品处理
2.4. 标准曲线及最低定量限
2.5. 系统精密度与准确度(回收率)
2.6. 稳定性试验
2.7. 基质效应
2.8. 方法在治疗药物监测中的应用
-
CA的代谢途径主要为Hoffman消除[7],Hoffman消除受温度和pH值的影响。有研究显示[8],将苯磺顺阿曲库铵注射液稀释于20%的甲醇中,室温下,10~15 min即可检测到大量降解产物N-甲基四氢罂粟碱和单季丙烯酸酯。血浆的pH值在7.4左右。我们发现,在CA血浆样本前处理过程中,如果不加入酸调节pH值,即使在-80 ℃的储存条件下,CA仍然会很快降解(结果未在文中体现)。为此,我们考察了不同比例的甲酸,最终确定了在样本前处理过程中加入2%的甲酸能够保证血浆中CA的稳定性。在既往报道中,多是加入一定浓度的硫酸来调控pH值[2-6]。硫酸为有机酸,对测定仪器尤其是质谱仪的离子源损害很大。同时,加入硫酸并不能保证室温条件下样本的长时间稳定(<4 h)[9]。本研究结果显示,加入2%的甲酸,室温下样本稳定性可保持在24 h以上。此外,我们的样本前处理过程并不需要经过离心-上清液真空浓缩或氮吹浓缩-复溶-离心取上清液等[2-6, 9]复杂过程,更为省时、省力。经验证,本研究建立的方法测定CA血浆样本的精密度、准确度和稳定性均符合方法学要求。该方法更加简便、准确、快速、稳定,可应用于临床CA治疗药物浓度的监测。






 本站查看
本站查看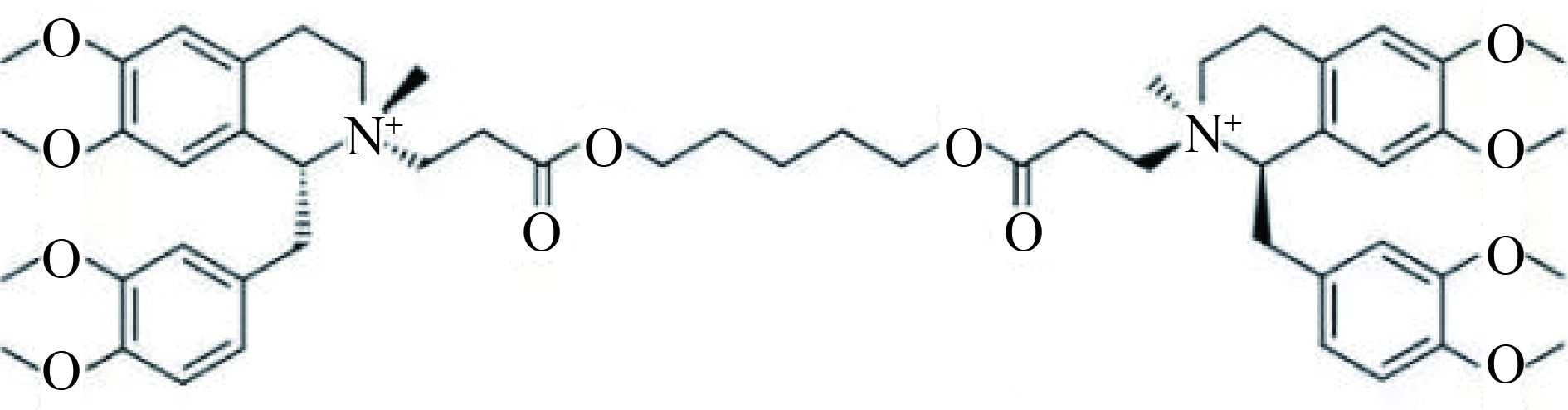


 DownLoad:
DownLoad: