-
微透析(microdialysis,MD)在体取样新技术[1-4]逐渐成为药动学研究中一种日趋成熟和实用的方法。由于其探针开发的多样性和微型化,定位更加准确,并利用极微量(一般若干微升)的透析装置实现在体、实时、在线取样和监测。能在微创条件下满足定量、定性、定位、连续动态取样分析等要求,且不破坏生物体内环境对靶器官或组织内的内源性和外源性物质进行取样[5-6],在采用微透析技术进行体内采样测定前,必须先在体外进行微透析探针增量、减量两种方法的回收率测定,以摸索出适合体内测定时的条件,以确保将微透析样品测得的浓度准确地折算为采样部位的组织浓度。
头孢拉定(cephradine)又称先锋霉素Ⅵ、头孢菌素Ⅵ等,其结构式见图1,是临床常用的第一代头孢菌素。头孢拉定耐酸,可以口服、吸收好、血药浓度较高,特点是耐β内酰胺酶,对耐药性金黄色葡萄球菌及其他多种对广谱抗生素耐药的杆菌等有迅速而可靠的杀菌作用,主要以原形经尿排泄,尿中浓度较高。临床主要用于呼吸道、泌尿道、皮肤和软组织等的感染,如支气管炎、肺炎、肾盂肾炎、膀胱炎、耳鼻咽喉感染、肠炎及痢疾等,也常用于预防外科术后感染[7]。本研究使用微透析技术与液质(LC-MS/MS)这一灵敏度高、检测速度快、前处理方便的分析方法相结合,测定头孢拉定微透析体外回收率,并考察回收率的影响因素,为进一步研究头孢拉定在前列腺组织、血液双位点的药动学提供可靠依据。
HTML
-
G-6410型高效液相色谱质谱联用仪(美国安捷伦科技有限公司);DL-180A型超声波清洗器(上海之信仪器有限公司);AG285型电子分析天平(Mettler Toledo仪器上海有限公司);微透析系统包括四通道微量注射泵,双通道微量收集器(瑞典CMA公司);微透析同心圆探针(瑞典CMA公司,CMA 20Elite,膜长10 mm)。
-
乙腈为色谱纯(美国Tedia公司);头孢拉定对照品(中国食品药品检定研究院,纯度88.4%);林格溶液(辰欣药业股份有限公司);水为三蒸水(海军军医大学附属长海医院制剂室);其他试剂均为分析纯。
1.1. 仪器
1.2. 药品与试剂
-
色谱柱:Agilent ZORBAX Extend-C18柱(2.1 mm × 100 mm,3.5 μm);流动相:乙腈-0.1%甲酸水溶液(20:80);流速:0.2 ml/min;进样量:1 μl;柱温:30 ℃。
-
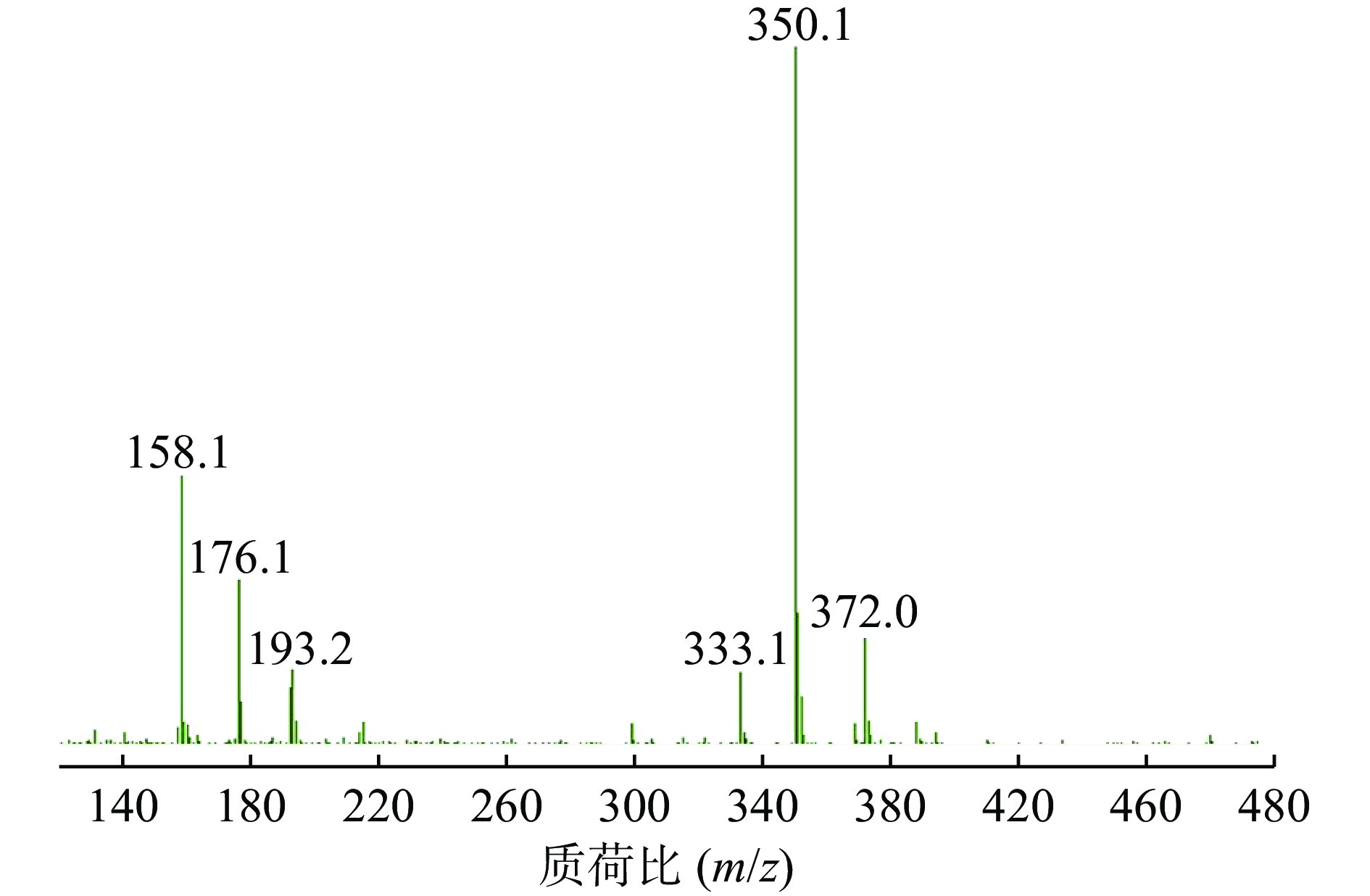
ESI+离子源,阳离子MRM扫描模式,干燥气体温度:350 ℃,干燥气流速:8 L/min,雾化压力:15 psi,裂解电压120 V,碰撞能量6 eV,定量离子对为m/z=350.1→176.1。
-
在Product Ion 模式下对碎片离子进行定量分析,如图3所示,其中m/z=176.1的碎片离子响应值最高且稳定。因此,选择的监测离子为m/z=176.1的碎片离子。
-
精密称取头孢拉定对照品适量溶于50 ml的棕色容量瓶中,加林格溶液超声溶解使成浓度为50 μg/ml的对照品储备液。将此溶液放于4 ℃冰箱中避光保存。
-
吸取适量“2.1.5”项下对照品储备液,用林格溶液稀释成系列浓度为20 000、10 000、2 000、500、100、25、10 ng/ml的头孢拉定标准品溶液,按上述液质条件进样。以质量浓度为横坐标(X,ng/ml),峰面积为纵坐标(Y)进行线性回归,绘制标准曲线,以加权平均数得回归方程为:Y = 34.096 2X + 150.818 5,r = 0.999,表明头孢拉定在10~20 000 ng/ml范围内线性关系良好,定量下限为10 ng/ml。
-
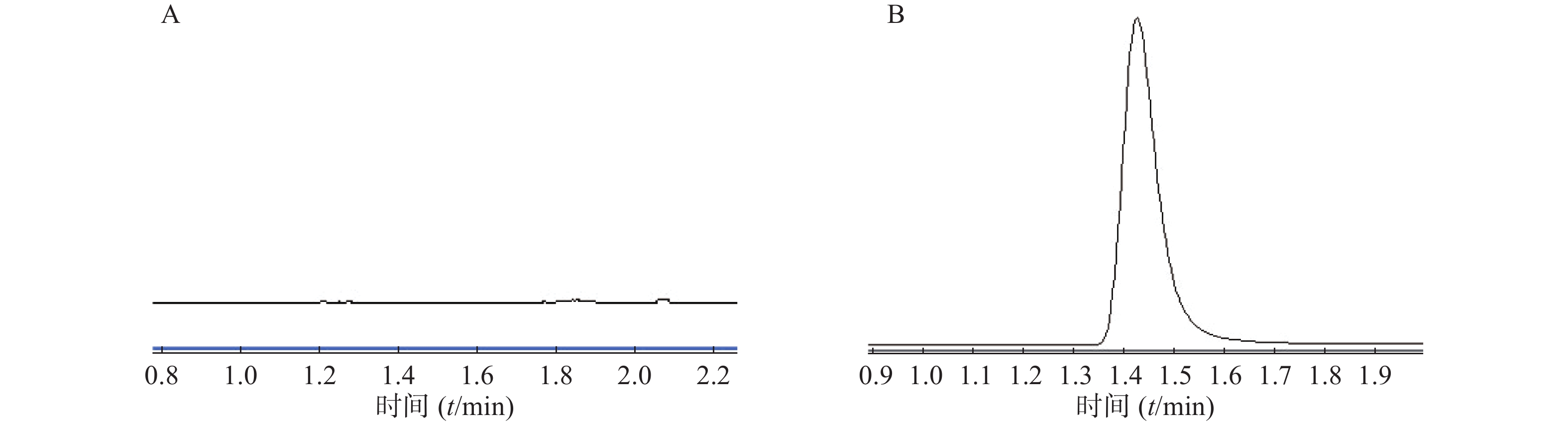
在本实验条件下,分别取林格溶液空白样品、微透析溶液对照品进样,记录峰面积。结果表明林格溶液对头孢拉定的测定无干扰,如图4。
-
同法配置浓度为25、500、10 000 ng/ml的低、中、高头孢拉定对照品溶液,同一日平行操作6次,连续测定3 d,按《中国药典》(2015年版)生物样品定量分析方法计算日内精密度、准确度和日间精密度、准确度。结果显示,头孢拉定对照品连续进样6次,连续测定3 d,计算结果见表1,说明方法的精密度、准确度良好。
标准浓度(ng/ml) 日内 日间 检测浓度(ng/ml) 精密度(%) 准确度(%) 检测浓度(ng/ml) 精密度(%) 准确度(%) 25 24.60±0.84 3.40 98.39 25.49±1.63 6.39 101.94 500 494.87±3.67 0.80 98.97 493.23±4.49 0.91 98.65 10 000 10 269.52±82.90 0.74 102.69 10 325.06±190.40 1.84 103.25 -
用林格溶液配制浓度为10 000、100 ng/ml的头孢拉定对照品溶液,分别在0、1、2、4、6、8、12 h进样测定,将结果与0 h进行比较。按《中国药典》(2015年版)生物样品定量分析方法计算所得的低、高浓度在室温25 ℃和4 ℃的稳定性,结果见表2,表明头孢拉定对照品溶液在12 h内稳定。
标准浓度(ng/ml) 检测浓度(%) 室温(25 ℃) 低温(4 ℃) 低浓度(100) 101.64±4.24 103.73±6.30 高浓度(10 000) 104.29±2.51 103.79±1.73 -
减量法:将同心圆探针(膜截留相对分子质量2 000)浸入装有空白林格溶液的200 ml微透析体外回收率校正实验反应瓶中,磁力搅拌器转速200 r/min,水浴温度设置为(37.0±0.5)℃,用含有一定头孢拉定浓度(C灌流液)的林格溶液以一定流速进行灌注。平衡0.5 h后收集一个空白样品,每种灌流速度下收集 5 份样品,每份30 μl,且每个流速之间平衡20 min。在所建立的液质条件下测定透析液中药物含量(C透析液)。减量法公式:
RL(%) = (C灌流液 – C透析液)/C灌流液 × 100%
增量法:将同心圆探针(膜截留相对分子质量2 000)浸入含有一定头孢拉定浓度(C灌流液)的200 ml微透析体外回收率校正实验反应瓶中,磁力搅拌器转速200 r/min,水浴温度设置为(37.0±0.5)℃。微透析灌流液为一定灌流速度的空白林格溶液,平衡0.5 h后收集样品,每更换一次灌流速度后平衡20 min,每种灌流速度下收集5份样品,每份30 μl,共收集15份。在所建立的液质条件下测定透析液中药物含量(C透析液)。增量法公式:
RG(%) = C透析液/C灌流液 × 100%
-
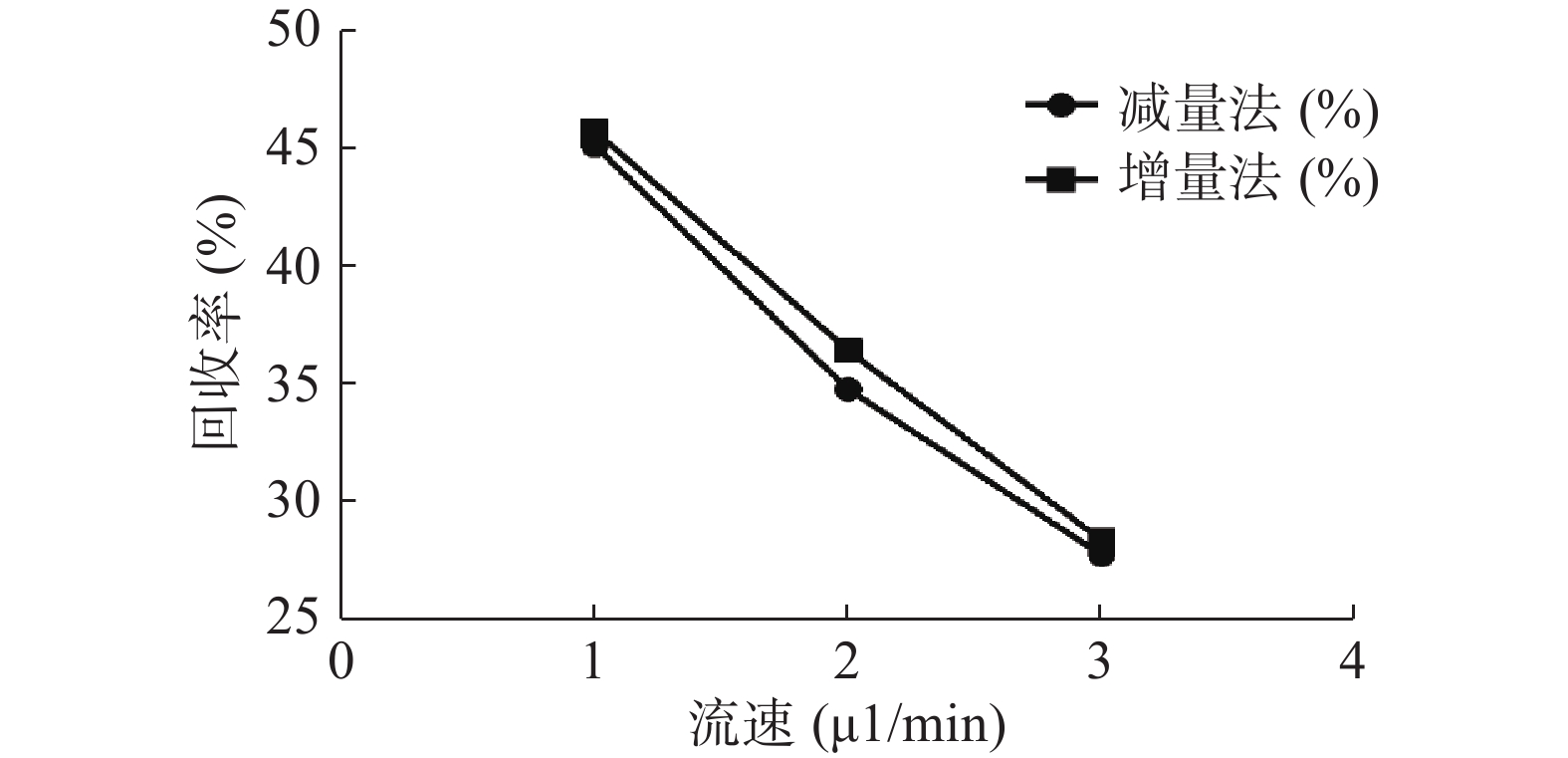
分别采用增量法、减量法研究流速对探针回收率的影响。以500 ng/ml的头孢拉定药物溶液,灌流速度依次为1.0、2.0、3.0 μl/min测定,并按公式计算探针回收率,结果见图5。结果显示,灌流速度从1 μl/min增至3 μl/min时,随着流速增加,回收率降低。而且,头孢拉定浓度为25、10 000 ng/ml 的回收率试验结果与此相似。
-
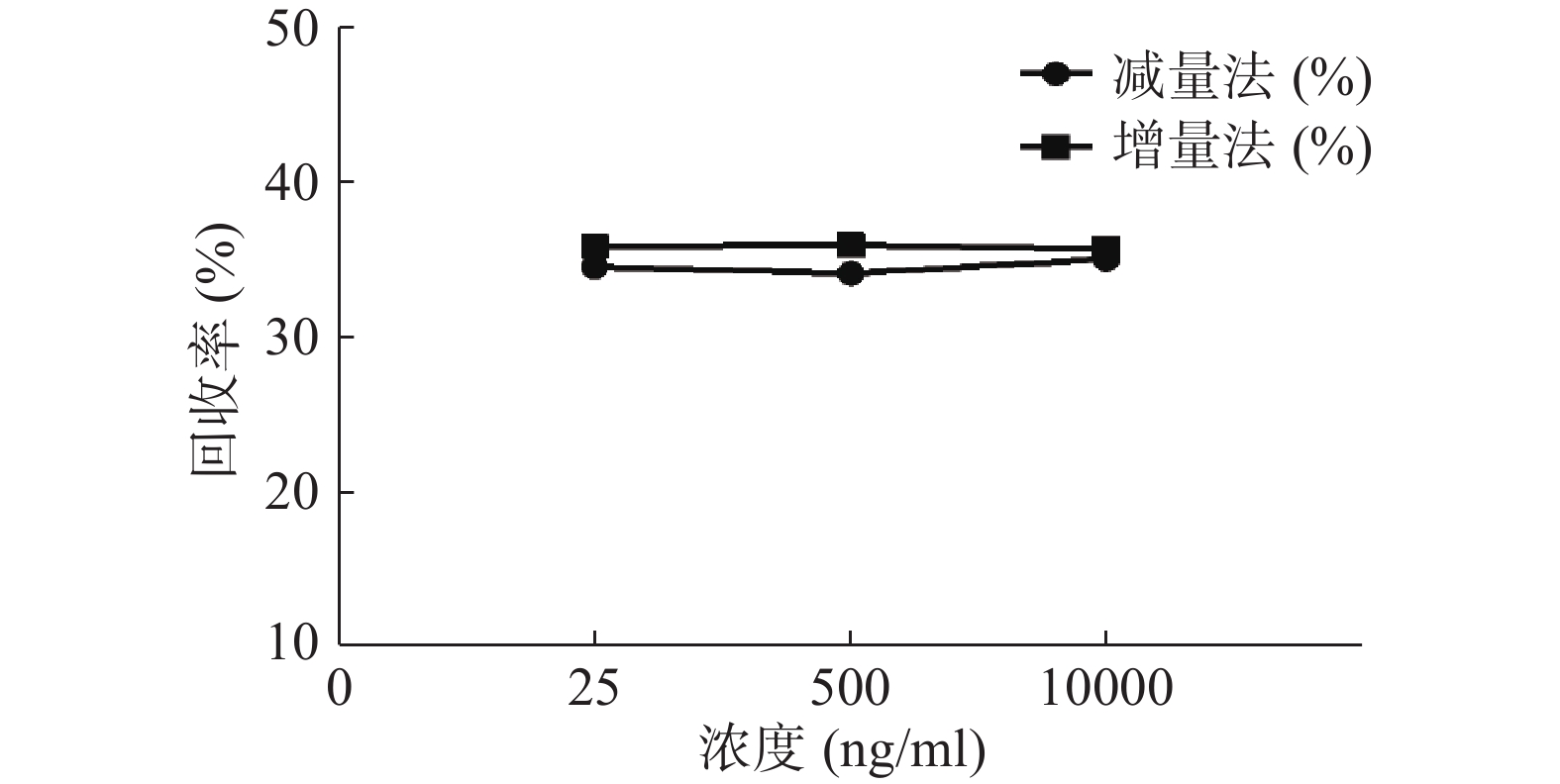
分别用增、减量法研究流速为2 μl/min,头孢拉定溶液浓度分别为25、500、10 000 ng/ml时的回收率,结果见图6。结果显示,当以2 μl/min的流速进行灌流时,同一探针不同浓度采用单因素方差分析测得的回收率均无显著性差异(P>0.05)。表明探针回收率与药物浓度高低无关。
2.1. 头孢拉定定量测定方法学
2.1.1. 色谱条件
2.1.2. 质谱条件
2.1.3. 准分子离子测定
2.1.4. 碎片离子扫描
2.1.5. 对照品溶液的配制
2.1.6. 线性关系考察
2.1.7. 专属性考察
2.1.8. 精密度、准确度考察
2.1.9. 稳定性考察
2.2. 头孢拉定微透析同心圆探针回收率及其影响因素
2.2.1. 微透析实验[9]
2.2.2. 灌流速度对头孢拉定体外回收率的影响
2.2.3. 浓度对探针体外回收率的影响
-
MD最初可追溯到20世纪60年代,当时不同类型的在体取样技术首次用于测定药物、介质、神经递质和代谢组织浓度[10-11],其原理是利用物质的扩散性和半透膜的选择透过性,探针头部半透膜位于组织内,且导管内液体与细胞内液体保持平衡,类似一个封闭的无孔毛细血管。这是一项为处在具有明显界限隔室里的游离型药物提供连续信息的微创技术。例如,成为测量细胞内和细胞外靶区浓度的标准工具。许多综述文章[12-17]已经证实MD是临床前和临床药学研发的有效工具。MD用于本研究的原理是基于血液-组织屏障的存在,屏障存在对血液浓度的依赖可能误导靶区浓度及药效学。在多年不断的研究中,人们发现血-前列腺屏障(BPB)的存在是影响前列腺炎药物治疗不可忽视的重要因素[18-20]。受采样和测定技术的限制,截止目前尚未明确BPB的具体部位和物质基础,故而很难研究其药物分布的特点和屏障作用的机制[21-23]。而MD有望解决前列腺组织特殊的部位和大小对技术上的高要求。有利于后期动物前列腺实验的开展。
微透析探针的回收率分为体内和体外,校正方法也有体内和体外之分。而体内校正应用反透析法即减量法的前提是探针的回收率与传递率近似相等,所以需要进行体外增、减量法的回收率试验。本实验结果可看出,体外回收率与流速成反比,与探针周围液体浓度无关,结果与多数报道相符[9, 24],由于待测部位的药物浓度是动态变化的,说明微透析技术可以用于体内药物浓度的测定。MD样品体积也与灌流速度成正比,在相同时间内流速越高体积越大,但是探针回收率也有所降低,这就要求分析方法检测限更低;而流速越低,透析液浓度越接近组织浓度,但为了收集足量样品体积用于检测,同时考虑时间分辨率问题须选择合适流速。此外,增、减量法所得到头孢拉定体外回收率结果相近,说明减量法即反透析法可以用于头孢拉定的体内回收率实验,并以此为体内药动学实验提供参考。








 DownLoad:
DownLoad: