-
心肌梗死是一种严重的心血管疾病,由于冠状动脉血供急剧减少或中断,使心肌持续性缺血缺氧以致坏死,损害心功能且可能导致心律失常、休克以及心力衰竭等严重后果,已成为威胁人类生命健康的重大疾病之一[1-2]。近几十年来,随着医疗技术的进步,再灌注心肌治疗可显著改善心梗患者的生存率,但由于心梗发生发展及转归过程极其复杂,使其在临床治疗中仍然面临许多挑战[3],因此构建合适的动物模型对探究人心肌梗死的发病机制和病理过程、评价药物疗效以及探索新的治疗方法至关重要。通过结扎冠状动脉模拟心肌缺血过程来建立心肌梗死的动物模型是目前广泛应用的较为成熟的方法[4-5]。以往更倾向于选择较大动物构建模型,随着基因工程技术的发展,基因工程小鼠成为炙手可热的研究工具,因此建立简便有效的小鼠心梗模型对心肌梗死疾病的深入研究有重要意义。但目前国内关于小鼠心梗模型构建方法的报道较少,缺乏一种比较便捷的模型制作方法和无创评价手段。本文基于Gao等[6]报道的心梗模型构建方法结合实际操作总结了一些经验。
HTML
-
SPF级C57BL/6J 10~12周龄雄性小鼠,共29只,体重为(29.65±5.35)g,购于海西普尔-必凯实验动物有限公司。实验动物均饲养在具有IVC系统的动物房[人工照明12 h;温度(23±2)℃;相对湿度40%~60%;噪音≤60 dB],自由饮食进水,在正式实验之前需适应饲养环境至少1周。动物实验方案与操作均遵守动物福利及“3R”原则。
-
异氟烷(河北一品制药);4%水合氯醛、2%TTC染液(批号:E110BA0007、AC29BA0025,BBI Life Science);0.9%NaCl溶液;4%多聚甲醛组织固定液(批号:154608,博光生物)。气体麻醉机(上海曼普生物科技有限公司);恒温垫;彩色扫描仪(BenQ,K802);MPA血压与心率分析系统(上海奥尔科特生物科技有限公司);显微外科手术器械。缝线(3-0,用于肌肉、皮肤缝合)、缝针;带线缝针(7-0,用于结扎冠状动脉)。
1.1. 实验动物
1.2. 试剂与仪器设备
-
所有手术器械使用前均高温蒸汽灭菌30 min。实验小鼠术前禁食12 h。按照麻醉机说明书连接好管路。将小鼠放进麻醉机的诱导箱,开启氧气调节气流量(0.25 MPa,1 L/min),调整麻醉药(异氟烷)浓度为5%,约1 min完成小鼠诱导麻醉,之后调整麻醉药浓度至2%,连接小鼠面罩并持续吸入。
-
小鼠仰卧位固定,左胸前手术区域剔除鼠毛并消毒。距胸骨左缘约1~2 mm皮肤处做长约1 cm纵向切口,切口处行垂直外翻褥式缝合预留缝线。逐层钝性分离胸壁肌肉,从第3或第4肋间隙快速进入胸腔,用止血钳撑开肋间隙,配合心脏跳动左手轻轻挤压使心脏从孔隙中弹出。在左心耳下缘1~2 mm、肺动脉圆锥旁0.5 mm处以7-0带线缝合针穿过冠状动脉前降支将其结扎,松紧适宜,控制进针深度(以隐约可见细针为宜)和行针宽度(2 mm左右)。由于不借助通气装置,要求开胸时间不要超过30 s,结扎操作尽量控制在10 s左右完成。结扎完成后轻柔地将心脏送回胸腔,挤压胸腔排出空气同时收紧结扎切口处预留缝线,完成手术。术中逐步调整麻药浓度至零,取下面罩后将小鼠置于恒温垫上约3~5 min即可复苏。
-
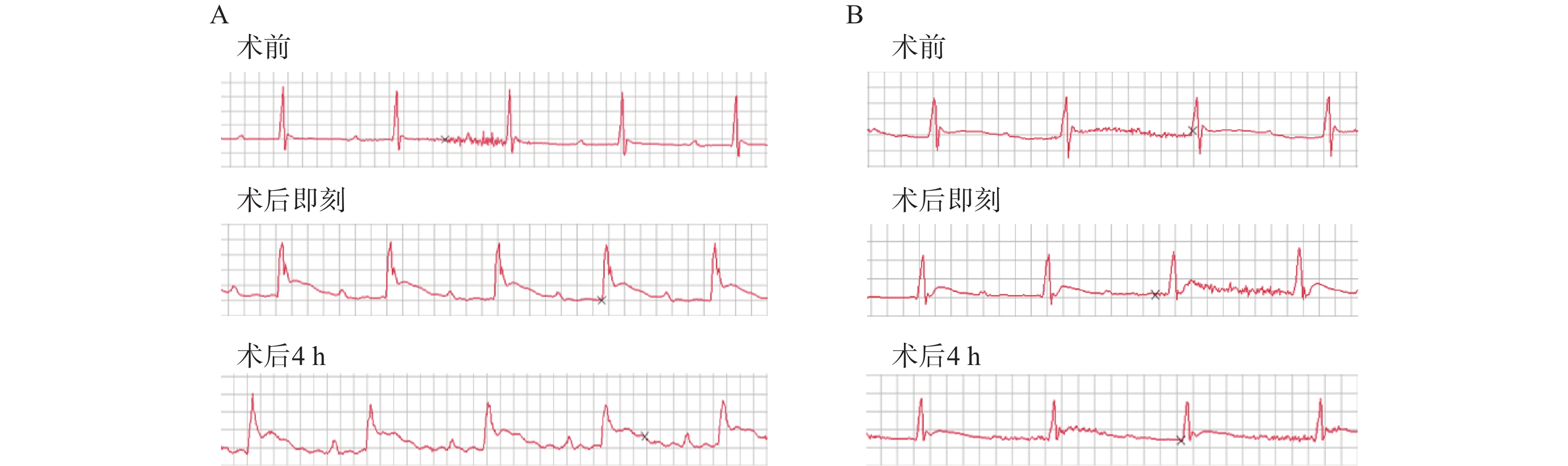
小鼠异氟烷吸入麻醉后,仰卧固定,按照仪器说明连接MPA系统心脏导联线,并将针电极分别固定在右上肢、左上肢,右下肢接地,避免周围磁场干扰。待基线平稳后,记录4~5个心动周期。实验中我们主要使用II导联观察心脏前下壁心梗发生的情况,与冠状动脉左前降支所供给的心肌区域相吻合。心电图导联的选择可以根据实验需求进行调整。
-
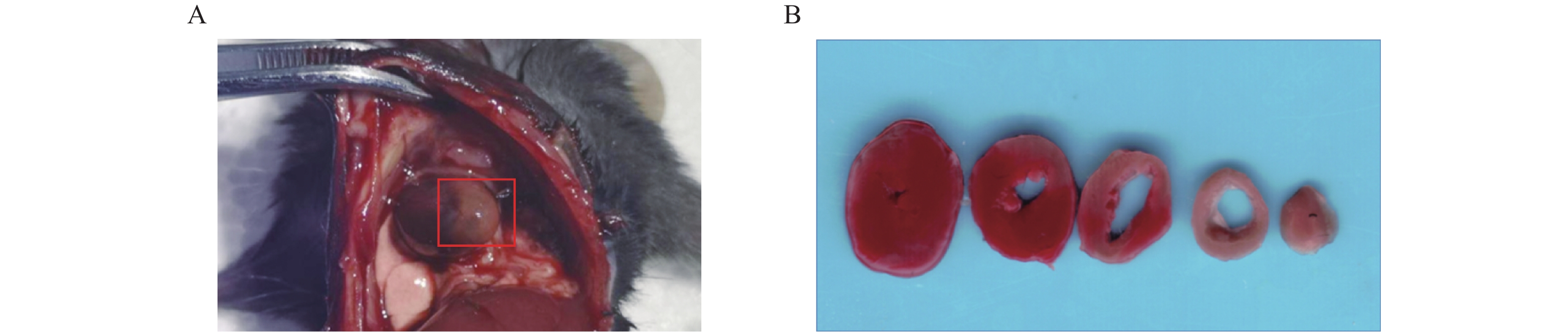
术后24 h对模型小鼠施行安乐死(4%水合氯醛,300 mg/kg),打开胸腔,肉眼观察心梗部位,并拍照记录。留取心脏标本置于生理盐水中,冲洗除去多余的血液,用滤纸吸干液体后修剪,仅保留全心室称重,记为M1(mg)。全心室心脏标本置于–20 ℃冷冻约30 min后,将心脏切成厚度为1 mm左右的薄片若干,经常规TTC染色后[7],进行扫描拍照。剪下白色梗死区域用滤纸吸干后称重,记为M2(mg),梗死区大小计算以百分比表示,即梗死大小(%)=(M2/M1)×100%。
-
数据均采用平均值±标准差(
$ {\rm{\bar X}} $ ±s)表示。
2.1. 术前准备及手术过程
2.1.1. 术前准备
2.1.2. 手术操作
2.2. 肢体导联心电图
2.3. TTC染色及梗死区域大小计算
2.4. 统计学方法
-
小鼠心梗模型总体成功率为79.3%(23/29)。其中,冠脉结扎术中死亡率为6.8%(2/29),1只由于穿刺过深导致心脏破裂出血,1只由于胸腔暴露时间过长导致气胸。术后早期(<4 h)死亡率为10.3%(3/29);2只解剖后发现胸腔内有大量积血,1只疑为发生不可逆转的致死型心律失常。此外,1只造模失败未发生心梗,术后24 h TTC染色未见明显梗死区,且术后即刻和术后4 h心电图均无ST段改变(表1)。
失败时段 比例(%) 原因分析 只数 解决方法 术中死亡 6.8 术中心脏破裂致死 1 控制穿刺深度和宽度 术中气胸致死 1 穿刺结扎迅速,快速闭合胸腔 术后死亡 10.3 术后胸腔出血致死 2 保证视野开阔,用纱布保护胸壁及肺,避免刺破大血管和肺脏 术后致死型心律失常致死 1 结扎动作迅速轻柔,减少对心脏的刺激 其他 3.4 术后未发生心梗 1 (造模失败) -
造模小鼠均施行术后即刻和术后4 h两次心电图检查,发现经24 h TTC染色后证实有心肌梗死发生的23只造模成功的小鼠中,两次心电图中均呈现出明显的ST段抬高以及变异T波(图1A),而造模失败的小鼠心电图则没有明显异常(图1B)。
-
术后24 h打开小鼠胸腔,肉眼可见左心室前下壁靠近心尖处呈灰白色(图2A)。取心脏标本经常规TTC染色后,可观察到梗死区域呈白色,而非梗死区域呈红色(图2B)。根据前文提到心梗区大小的计算方法,本实验中,心梗模型梗死区大小最大可达40%,几乎累及整个左心室;最小为17%,主要集中在心尖部位;梗死区平均大小为(28±6)%(见表2)。
项目 体重/g 全心室重量(M1)/mg 梗死心肌重量(M2)/mg 梗死大小(M2/M1)/(%) 总计($ {\rm{\bar X}} $±s) 29.65±5.35 76±13 21.91±7.06 28±6
3.1. 小鼠心梗模型构建成功率
3.2. 无创肢体导联心电图变化
3.3. 心脏梗死区域TTC染色
-
建立疾病的实验动物模型常常是研究工作至关重要的一步,需要考虑多方面的因素,使疾病本身特征和研究目的与所建立的动物模型达到尽可能地一致。首先,实验动物的选择要考虑各实验室以及实验者自身的条件。就心梗模型而言,大动物(猪、犬、兔等)因其心脏在形态大小、解剖结构以及生理条件上与人类更为相似,构建模型成功率相对较高,通常用于病理生理过程以及药物治疗疗效等研究。小动物生命力弱、手术耐受性差,导致模型建立具有一定难度,但由于其能够建立相关基因工程动物形成较为一致的品系,对于深入研究心肌梗死病理及治疗相关机制有重要意义。目前心梗治疗研究不断深入,基于心肌细胞不可再生的特性,移植治疗、干细胞治疗、基因治疗等多种治疗手段及相关机制研究成为心梗治疗方案的新方向[8-10],建立一种稳定性强、成功率高的小鼠心梗模型是非常必要的。因此,笔者结合小鼠自身特点,在以往大动物模型的基础上,比较成功地建立了小鼠的心梗模型。
目前建立心肌梗死动物模型的方法有多种,包括:冠脉结扎法、药物法、球囊堵闭法、栓塞法以及血栓形成法等。其中药物法主要使用垂体后叶素等诱发血管痉挛促使心肌缺血梗死,但此法很难明确发生梗塞的动脉且效果具有不确定性;后三种方法可直接形成冠脉闭塞,但需要借助心导管技术和冠脉造影等影像学技术,且小鼠细小的冠状动脉给操作增加了难度[11]。而冠状动脉结扎法[12]操作简单、血管阻塞明确,比较符合心梗发生的病理过程,能较好的实现临床转化,因此本实验主要采用此法建立小鼠心梗模型。实验过程中,采用5%异氟烷诱导后联合2%异氟烷面罩持续吸入麻醉的方法,不同于传统的腹腔麻醉联合气管插管通气的方法[13],避免了气管插管和机械通气可能产生的组织损伤,同时也提高了麻醉的安全性和手术效率。但是,此法要求实验者在短时间内完成冠脉结扎术,尽快完成冠脉结扎并闭合胸壁,降低长时间暴露胸腔可能导致心律失常、气胸等致死风险。术中需要密切关注小鼠状况以便及时调整麻药浓度,从而促进小鼠的术后复苏。除了安全有效的麻醉方法之外,冠脉结扎位置的选择至关重要,由于小鼠血管细小,侧枝循环丰富且走行不易观察,因此要求实验者操作精确,既要保证能够引起足够面积的心肌梗死,又要避免梗死面积过大而导致死亡,尽量在同一高度结扎形成较为一致的模型;此外,小鼠心室壁较薄,操作者需要严格控制穿刺深度,避免穿刺过深直接刺破室壁造成大出血。
心梗模型的评价方法可根据实验动物的生存状态分为活体检测和尸检。前者主要包括冠状动脉造影荧光微粒注射、MRI、PET和超声心动图等[14-15],这些方法可以获得冠脉血供状况从而间接评估心肌梗死,但设备要求高、价格昂贵,检测操作复杂;后者则主要是染料染色法包括TTC、依文思蓝、Masson三色法等,可以获得心肌梗死的直接结果。心电图通常是临床心肌梗死病人首选的实验室检查。然而,在动物心梗模型的评价中,人们往往忽视这一最为简便快捷方法的应用。在本实验的模型构建中,笔者采用术后即刻和术后4 h心电图检查两个指标来评估小鼠心梗情况。首先,在术后立刻对模型鼠施行心电图检查有助于帮助我们判断冠脉结扎的初期效果,以及发现潜在的心律异常改变。其次,考虑到心肌梗死特异性标志物之一——肌钙蛋白(cTn),一般在心肌坏死后3~4 h开始升高[16],我们又选择术后4 h的心电图检查,再次对模型小鼠心梗发生情况进行判断,同时,排除由于手术对心脏刺激可能造成的假阳性结果,并利用术后24 h TTC病理染色来验证心电图评估心梗发生的准确情况。最终,发现心电图评价心梗发生的准确性较高,且具有操作简便、结果获取迅速、不受场地时间限制、经济成本低等特点,故而在模型制备结果的评价中较其他活体检测方法有明显优势。
本研究成功构建了小鼠心肌梗死模型并利用TTC染色法明确区域。同时观察到,心梗模型小鼠早期心电图呈现出明显ST段抬高可以作为心梗发生的可靠依据,即心电图可以作为小鼠心梗模型早期快速无创评价的可靠方法。因此,本实验建立的关于小鼠心肌梗死模型的高效制作方法和无创评价手段为确切研究心梗病理生理及治疗机制提供了良好选择。









 DownLoad:
DownLoad: