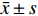-
啮齿类动物暴露于各种环境和实验等应激刺激条件下,产生许多行为和生理性应激反应[1-2]。应激刺激可致啮齿类动物痛阈升高,痛觉敏感性降低而诱发镇痛,即应激镇痛。测定痛阈的方法有压尾法、甩尾法、热板法和压脚法等[1]。根据应激刺激的特点(如应激源,持续时间,强度和时间模式),镇痛可能由内源性阿片系统或其他非阿片的激素和神经生化机制调解[2-3]。
旋转刺激可诱发啮齿类动物发生晕动病症状,例如异食癖,条件性厌食症,自发活动减少和激素水平变化等[4-6]。肾上腺酮等激素水平的升高,提示某些前庭刺激,如旋转亦可诱发啮齿类动物应激镇痛反应,引起应激镇痛的旋转亦是诱发啮齿类动物晕动病的有效前庭刺激条件。可见,以角加速度为主的平面旋转刺激,可诱发小鼠晕动病和应激镇痛反应。因此,研究旋转诱发的晕动病与应激镇痛之间功能联系的机制非常有意义[7-8]。二者都是通过相同的前庭刺激引起的,反应程度都依赖于应激刺激强度、类型和持续时间等。而且,重复的旋转刺激易致小鼠对镇痛耐受和晕动病的习服。
本实验以热板潜伏期为指标,在以角加速度为主的水平旋转刺激下,观察了化学迷路切除对小鼠旋转诱发的应激镇痛和吗啡镇痛的影响。
-
吗啡原料(青海制药厂);纳洛酮原料(北京四环药厂);对氨基苯胂酸(Sigma公司)。
-
健康昆明种小鼠,雌性,体重:18~22 g。小鼠为清洁级,实验动物质量合格证号:SCXK(沪)2002-0002,由复旦大学医学院实验动物部提供。
-
小鼠旋转仪:由控制装置和旋转装置两部分组成。控制装置可调节并显示旋转装置的旋转速度、旋转时间和变速周期。本实验选择转速250 r/min,旋转时间1 min,变速周期为每旋转15 s停止5 s。
旋转装置位于控制装置上方,为一可旋转的方形有机玻璃板(21 cm×21 cm×0.2 cm),旋转转盘的对角线的四角排列着4个可以容纳小鼠的三角形有机玻璃小盒,小盒中心距离旋转轴心均为10 cm。在250 r/min转速下,旋转时小鼠头朝向离心端,旋转半径为10 cm。
热板仪:由电热恒温水浴锅和热板组成。电热恒温水浴锅可程控设置,并显示水浴温度,温度控制精确到0.1 ℃。热板为紫铜板焊接而成的25 cm×14 cm×7 cm(长×宽×高)的槽形体,底部嵌入水浴水面下5 cm,以保证热板受热充分而均匀,由于浮力使热板槽卡紧在水浴锅中。
-
应用小鼠旋转仪和热板仪。热板的水浴指示温度为(55.0±0.5)℃。实验时室温维持在23~25 ℃,环境湿度和照明度等应控制相对恒定,减小系统误差。实验时旋转刺激前先测定2次小鼠热板反应时间(即放入热板至首次舔后足时间)取其平均值作为基础潜伏期,挑选基础潜伏期在10~20 s的小鼠作为实验动物,2次测定间隔至少5 min。旋转后热板反应时间在30~50 s较合适。如舔后足潜伏期超过60 s,应中止热板刺激,以免烫伤足底。
-
取雌性小鼠32只,随机分为4组,每组8只:①吗啡(5 mg/kg,sc)+生理盐水(10 ml/kg,ip);②吗啡(5 mg/kg,sc)+纳洛酮(4 mg/kg,ip);③生理盐水(10 ml/kg,ip)+旋转刺激;④纳洛酮(4 mg/kg,ip)+旋转刺激。①②组小鼠给药15 min后即刻测定热板潜伏期;③④组小鼠给药15 min后开始旋转,旋转结束后即刻测定热板潜伏期。
-
取雌性小鼠20只,随机分为2组,每组10只,即生理盐水对照组(10 ml/kg,sc)和吗啡组(5 mg/kg,sc)。每天给药2次(上午9时,下午4时),连续给药7 d。d0、d1、d3、d5和d7下午给药15 min后分别测定小鼠热板潜伏期。首次给药前(d0)和末次给药后(d7)15 min分别测定旋转后小鼠热板潜伏期。
-
取雌性小鼠16只,随机分为前庭损伤组(对氨基苯胂酸钠)和假损伤组(生理盐水),每组8只。取0.3 mol/L的NaHCO3溶液加至100 mg/ml的对氨基苯胂酸溶液中,调节pH中性即可形成对氨基苯胂酸钠溶液。动物经戊巴比妥钠麻醉后,注射针穿过鼓膜向鼓室内注射对氨基苯胂酸钠溶液或生理盐水0.04~0.06 ml,注射完毕后用火棉胶紧紧塞住以防药液渗漏。监测动物直到麻醉苏醒可放入饲养笼恢复3~5 d。
-
将小鼠置于去芯的50 ml注射器外管中使其呈仰卧位。正常小鼠能感受体位颠倒,并且在数秒内翻正颠倒的体位;前庭损伤小鼠则难以感受颠倒的体位而不能翻转或体位翻正时间延长。实验中观察并记录小鼠翻正反射恢复时间。
-
将小鼠置于20 cm × 40 cm的盛水容器中,温度控制在(23 ± 2)℃,观察小鼠在60 s内头部露出水平面游泳的时间。正常动物可在60 s内一直保持头部露出水面游泳,而前庭损伤者头部常难以浮出水面。
-
前庭功能评价后,先测定2次小鼠热板反应时间取其平均值作为基础潜伏期。然后,测定前庭损伤和假损伤小鼠旋转后热板潜伏期。
最后,观察迷路切除对吗啡镇痛作用的影响。给予吗啡(5 mg/kg,sc)15 min后测定前庭损伤和假损伤小鼠热板潜伏期。
-
数据以均数±标准差(
$ \bar{x}\pm s $ )表示。采用SPSS 13.0统计软件进行多元方差分析(MANOVA)和单因素方差分析(LSD法)、t检验,α=0.05视为检验水准。 -
同时注射吗啡和纳洛酮15 min后,小鼠热板潜伏期显著低于生理盐水对照组(P<0.01),纳洛酮显著降低了吗啡组小鼠热板潜伏期。旋转刺激后,纳洛酮组小鼠热板潜伏期与生理盐水组比较无统计学意义(P>0.05),结果见图1。
本实验结果表明,阿片受体拮抗剂纳洛酮可拮抗吗啡的镇痛作用,但不能拮抗旋转诱发的应激镇痛作用,提示旋转诱发的热板潜伏期延长与吗啡镇痛作用机制可能不同,或旋转诱发的小鼠热板潜伏期延长是可能通过非阿片系统起作用的。
-
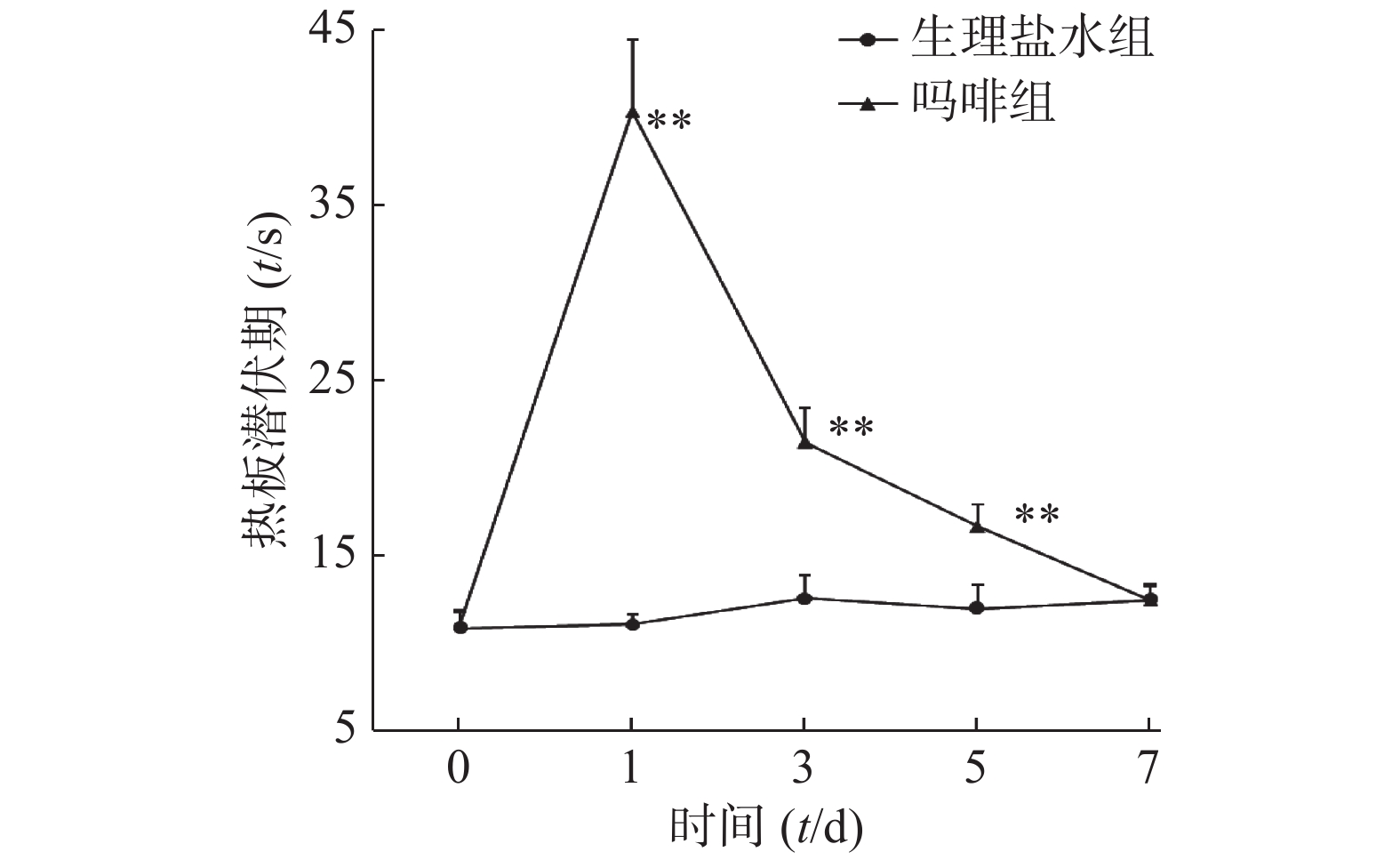
图2所示,吗啡组小鼠随给药天数增加,热板潜伏期逐渐缩短。d1、d3和d5吗啡组小鼠热板潜伏期显著高于生理盐水组(P<0.01);d7末次给药后2组小鼠热板潜伏期无统计学差异(P>0.05)。可见,吗啡组小鼠于d7已形成吗啡耐受。
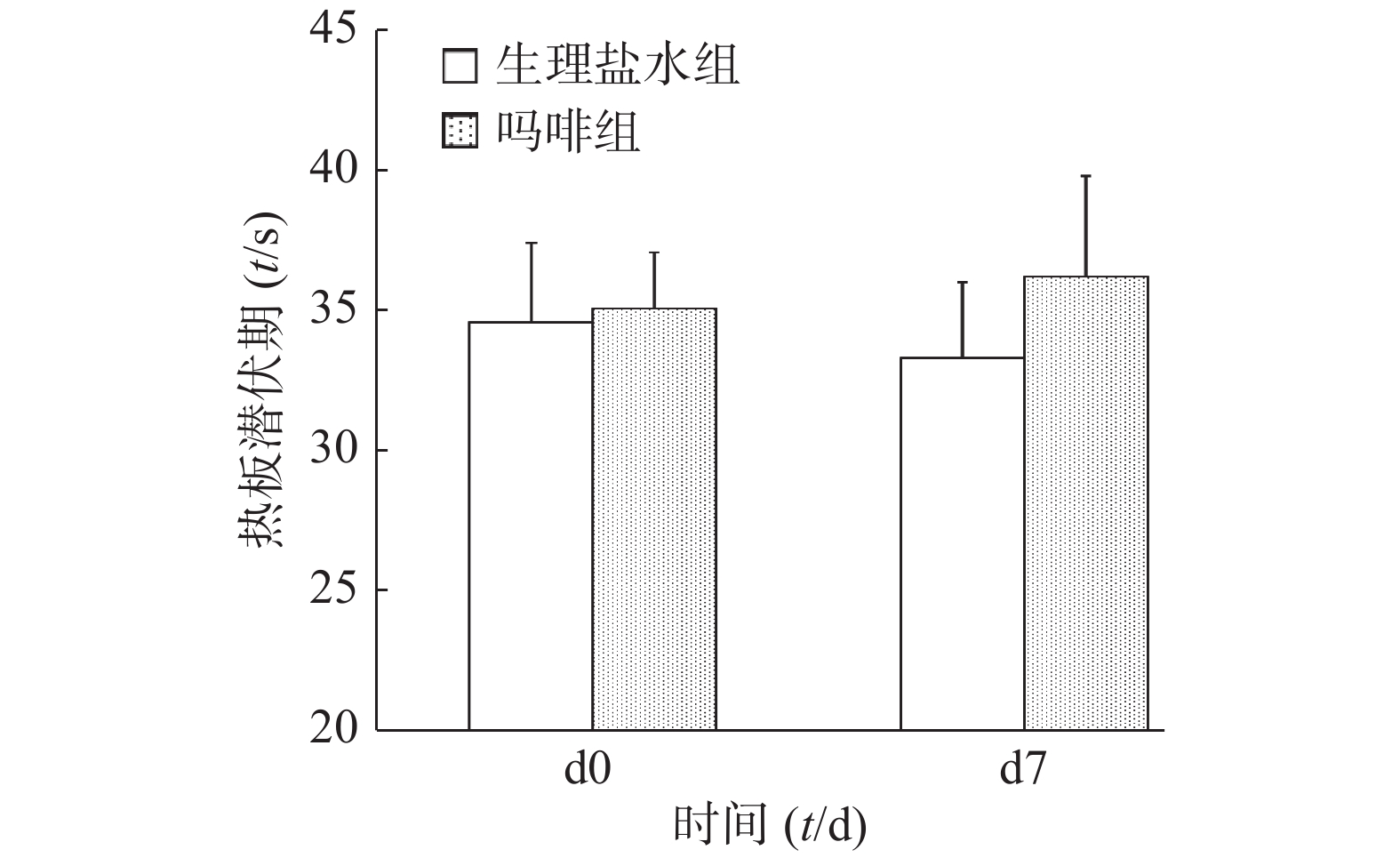
图3所示,给药前(d0)和末次给药后(d7),吗啡组和生理盐水组小鼠旋转后热板潜伏期均无统计学差异(P>0.05)。结果提示,吗啡镇痛和旋转诱发的应激镇痛无交叉耐受性。
-
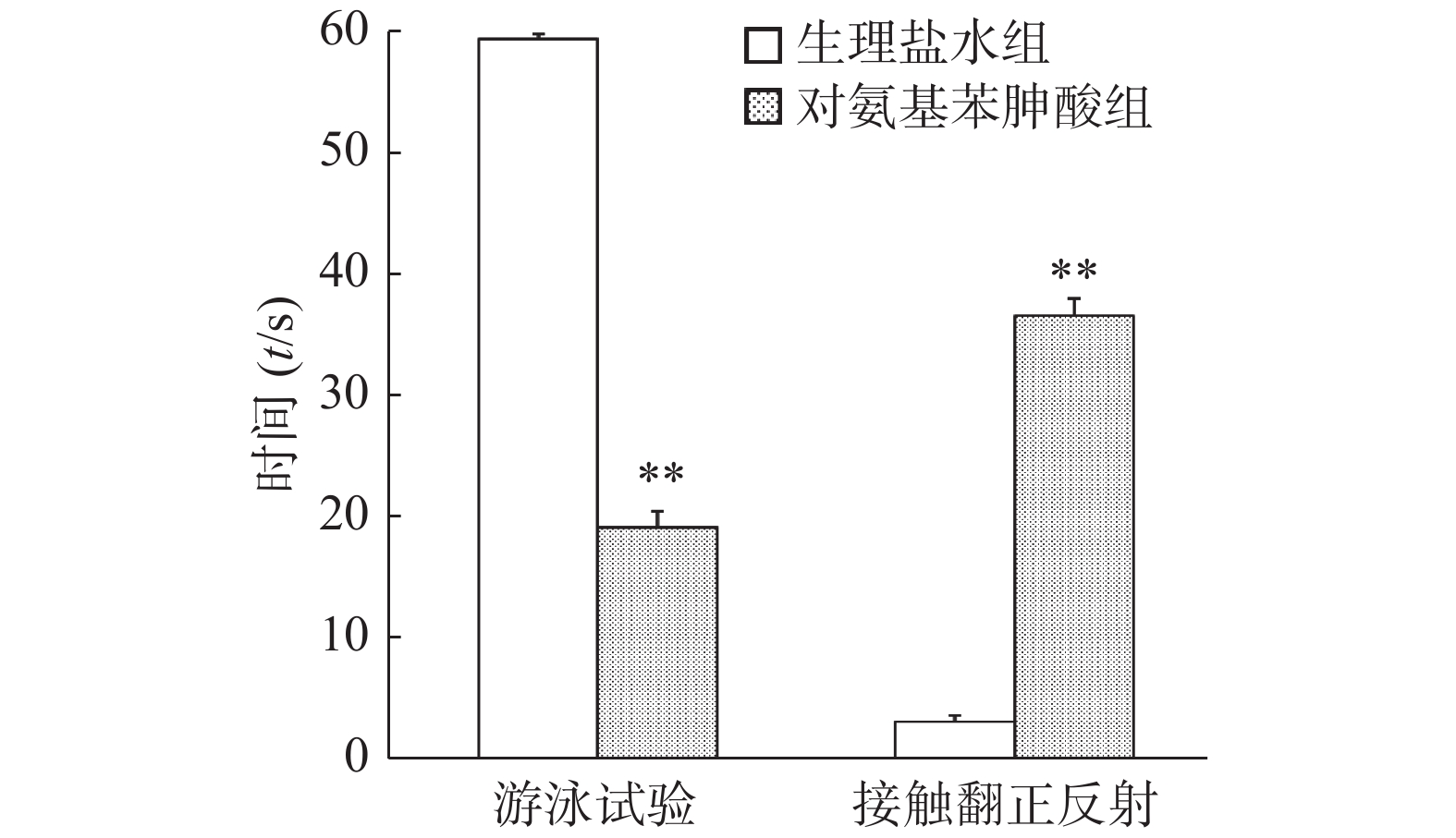
接触翻正反射试验中,给予对氨基苯胂酸溶液组小鼠在注射器中翻正所需时间显著高于生理盐水组(P<0.01)。游泳试验中,给予生理盐水组小鼠在60 s内一直保持头部露出水面游泳,而给予对氨基苯胂酸组小鼠身体在水中螺旋翻滚,头部难以浮出水面,两组小鼠60 s内头部露出水面游泳时间的差别具有统计学意义(P< 0.01),结果见图4。
-
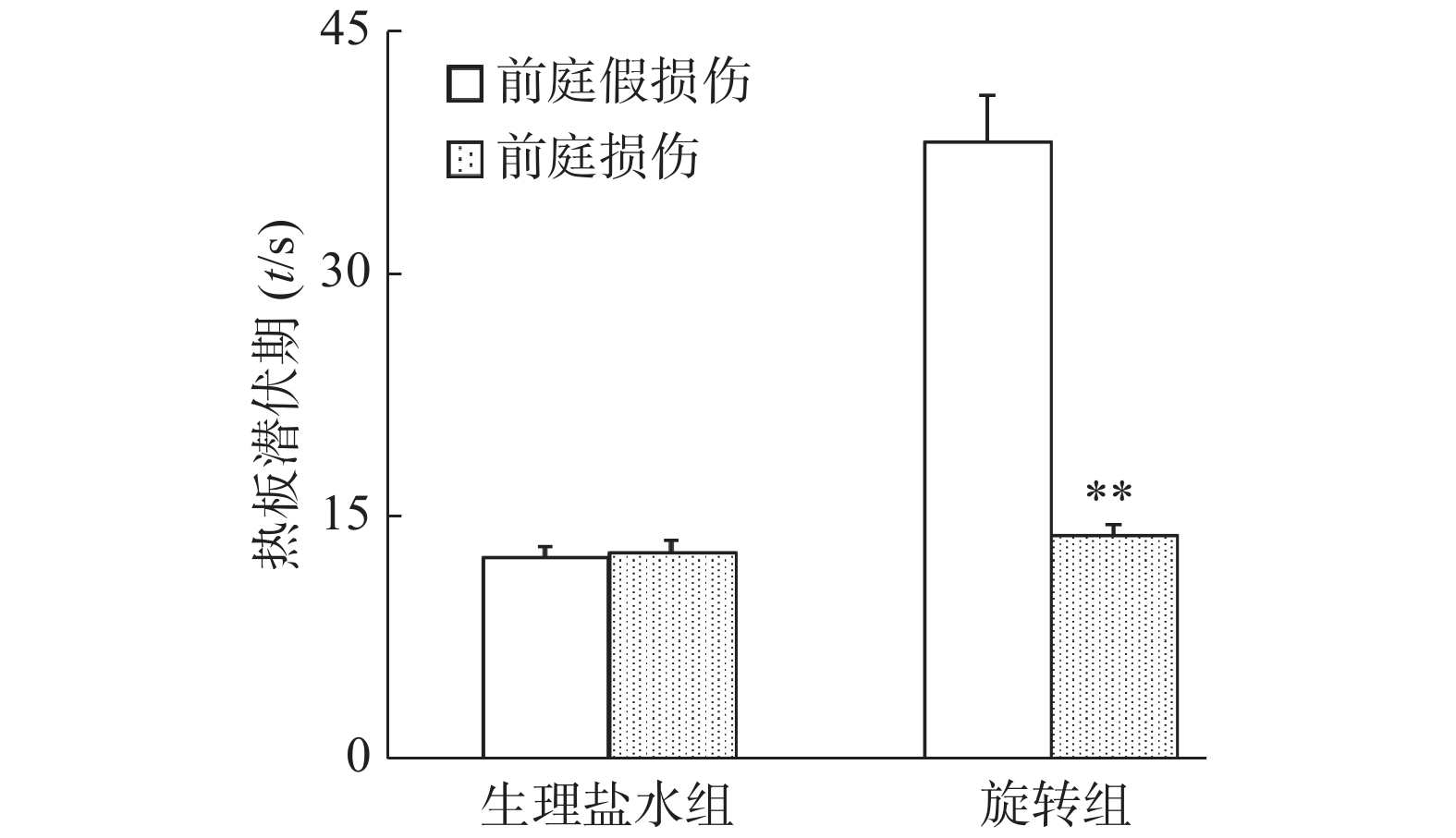
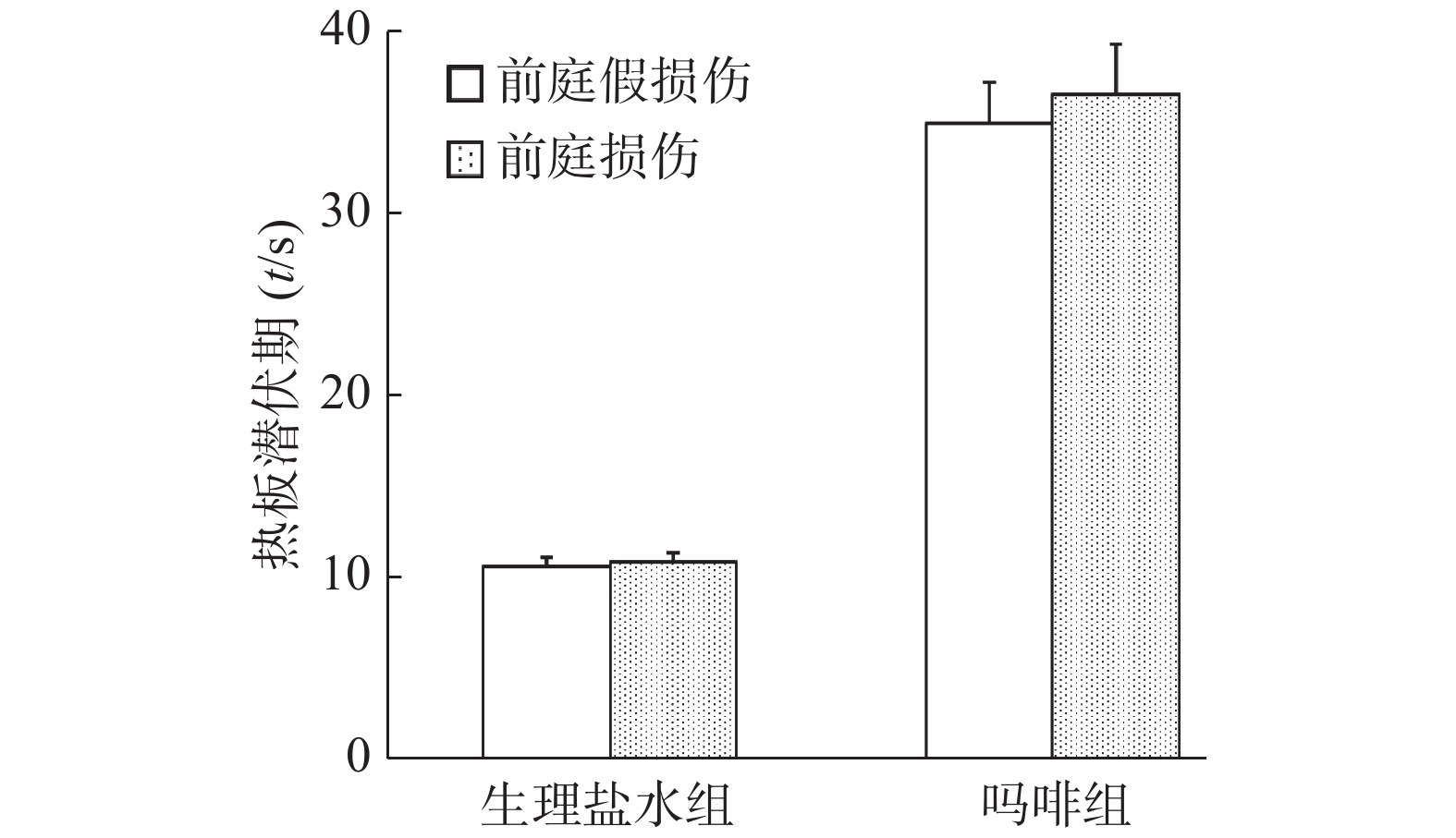
旋转刺激后,假损伤组小鼠自发活动减少而旋转行为增加,而前庭损伤组小鼠自发活动在旋转前后无明显变化。从图5显示,旋转前假损伤组和前庭损伤组小鼠基础潜伏期无统计学意义(P>0.05);而旋转后前庭损伤组小鼠热板潜伏期显著降低(P<0.01),其数值几乎接近旋转前的基础潜伏期水平(旋转前12.8 s,旋转后13.9 s),而假损伤组小鼠旋转后热板潜伏期为38.3 s(旋转前12.5 s)。结果提示,化学迷路切除可能完全阻滞了旋转诱发的小鼠应激镇痛作用。
注射吗啡15 min后,前庭假损伤组和前庭损伤组小鼠热板潜伏期无统计学意义(P>0.05,图6)。结果提示,化学迷路切除对小鼠吗啡镇痛作用无明显影响。
-
应激是机体在受到各种强烈因素(即应激原)剌激时出现的以交感神经兴奋和垂体-肾上腺皮质分泌增多为主的一系列神经内分泌反应,并由此而引起的各种功能和代谢的改变。一定强度的任何躯体的或情绪的剌激,都可以成为应激原,如创伤、缺氧、疼痛等。啮齿类动物如大鼠或小鼠在电击足底,冷水游泳,固定,掐尾巴,离子辐射,或离心旋转等应激刺激下,痛觉敏感性均减弱,这一现象被称为“应激镇痛”[1, 6-7]。
阿片受体拮抗剂纳洛酮可拮抗吗啡对小鼠的镇痛作用,使小鼠热板潜伏期显著下降。可见,吗啡镇痛是通过阿片系统介导的。而旋转刺激诱发的痛阈上升不能被纳洛酮阻断,即旋转后纳洛酮和生理盐水对照组小鼠热板潜伏期间无显著差异。结果提示,旋转刺激诱发的应激镇痛可能是非阿片系统介导的。此外,旋转刺激对吗啡耐受小鼠应激镇痛的影响实验结果也显示,吗啡耐受小鼠和生理盐水对照小鼠旋转刺激后的热板潜伏期无显著差异,表明吗啡镇痛和旋转诱发的应激镇痛无交叉耐受性,证实旋转诱发的应激镇痛是非阿片系统介导的。因此,旋转诱发的应激镇痛与吗啡镇痛的作用机制可能不同。
诱发晕动病的旋转等前庭刺激条件也可以诱发小鼠应激镇痛,提示旋转刺激诱发的应激镇痛和晕动病效应可能存在共同的中枢机制。前庭器官在晕动病的发生中起着重要作用[12-13]。本实验中小鼠旋转后热板潜伏期显著高于旋转前的热板潜伏期,提示该前庭刺激诱发了应激镇痛,前庭器官在旋转诱发的应激镇痛中也起重要作用。
本实验进一步探讨了前庭器官在小鼠旋转诱发的应激镇痛中的作用。采用内耳注射对氨基苯胂酸溶液对小鼠施行化学迷路切除术[12-13]。游泳行为和翻正反射评估的是半规管和耳石器功能,是能够全面评价前庭功能的两个重要参数。评价小鼠前庭功能的小鼠接触翻正反射和游泳试验结果显示,对氨基苯胂酸组小鼠接触翻正反射时间显著高于生理盐水对照组,而头部露出水面游泳时间显著低于生理盐水对照组,提示对氨基苯胂酸成功损伤小鼠前庭功能,化学迷路切除术成功。
化学迷路切除小鼠旋转后热板潜伏期显著降低,且与旋转前热板潜伏期无明显差异;而正常对照小鼠旋转后热板潜伏期显著高于旋转前,表明化学迷路切除可能完全抑制或阻滞了旋转诱发的应激镇痛。另外,小鼠化学迷路切除与否,对吗啡的镇痛作用无影响,进一步提示旋转诱发的应激镇痛与吗啡镇痛作用机制可能不同,前庭器官在旋转诱发的应激镇痛中作用明显。
总之,动物行为学实验表明,前庭器官在旋转诱发的应激镇痛中起重要作用,而前庭系统究竟如何调节旋转诱发的应激镇痛有待进一步的验证和研究。
Effects of chemical labyrinthectomy on stress analgesia induced by rotation in mice
-
摘要:
目的 探讨前庭器官在旋转诱发的小鼠应激镇痛中的作用。 方法 雌性小鼠随机分为吗啡组和旋转组,每组小鼠腹腔注射纳洛酮或生理盐水15 min后,观察给予吗啡或旋转(转速250 r/min,时间1 min,每旋转15 s暂停5 s)刺激后的热板潜伏期。另取小鼠连续7 d皮下注射吗啡形成耐受后,观察吗啡耐受小鼠旋转后的热板潜伏期。最后,内耳注射对氨基苯胂酸损伤小鼠前庭器官,观察化学迷路切除小鼠旋转后的热板潜伏期。 结果 与生理盐水组比较,纳洛酮组小鼠旋转后热板潜伏期无明显变化(P>0.05),皮下注射吗啡后热板潜伏期显著下降(P<0.05)。吗啡耐受小鼠旋转后热板潜伏期与生理盐水组比较无明显变化(P>0.05)。内耳注射对氨基苯胂酸后,小鼠接触翻正反射恢复时间显著增加、游泳能力显著下降(P<0.05),且化学迷路切除小鼠旋转后热板潜伏期显著缩短(P<0.05)。 结论 化学迷路切除完全阻滞了旋转诱发的小鼠应激镇痛。前庭器官在旋转诱发的应激镇痛中起重要作用,且该应激镇痛可能由非阿片系统介导。 Abstract:Objective To investigate the role of vestibular organs on stress analgesia induced by rotation in mice. Methods Female mice were randomly divided into morphine group and rotation group. After 15 minutes of intraperitoneal injection of naloxone or normal saline, the hot plate latency of mice in each group was observed following morphine injection or rotation (250 r/min, 15 s on with 5 s off). After subcutaneous injecting morphine for 7 consecutive days, tolerance was formed and the hot plate latency in morphine-tolerant mice after rotation was observed. P-aminophenylarsonic acid was injected into the inner ear to damage the vestibular organs of the mice and the hot plate latency was observed in chemically labyrinthectomy mice. Results Compared with the normal saline group, the hot plate latency of mice in the naloxone group did not change significantly after rotation (P>0.05), and the hot plate latency decreased significantly after subcutaneous injection of morphine (P<0.05). The morphine-tolerant mice had no significant change in the hot plate latency after rotation compared with the normal saline group (P>0.05). After injection of p-aminophenylarsonic acid into the inner ear, the recovery time of the righting reflex in mice was significantly increased, and the swimming ability was significantly reduced (P<0.05), and the hot plate latency of mice with chemical labyrinthectomy was significantly shortened after rotation (P<0.05). Conclusion Chemical labyrinthectomy completely blocked the rotation-induced stress analgesia in mice. Vestibular organs play an important role in rotation-induced stress analgesia, and this stress analgesia may be mediated by a non-opioid system. -
Key words:
- rotation /
- morphine /
- stress-induced analgesia /
- vestibular organs
-
[1] HAYES R L, BENNETT G J, NEWLON P G, et al. Behavioral and physiological studies of non-narcotic analgesia in the rat elicited by certain environmental stimuli[J]. Brain Res,1978,155(1):69-90. doi: 10.1016/0006-8993(78)90306-2 [2] BODNAR R J, KELLY D D, STEINER S S, et al. Stress-produced analgesia and morphine-produced analgesia: lack of cross-tolerance[J]. Pharmacol Biochem Behav,1978,8(6):661-666. doi: 10.1016/0091-3057(78)90263-0 [3] BODNAR R J, KELLY D D, SPIAGGIA A, et al. Dose-dependent reductions by naloxone of analgesia induced by cold-water stress[J]. Pharmacol Biochem Behav,1978,8(6):667-672. doi: 10.1016/0091-3057(78)90264-2 [4] OSSENKOPP K P, RABI Y J, ECKEL L A, et al. Reductions in body temperature and spontaneous activity in rats exposed to horizontal rotation: abolition following chemical labyrinthectomy[J]. Physiol Behav,1994,56(2):319-324. doi: 10.1016/0031-9384(94)90201-1 [5] FOX R A, LAUBER A H, DAUNTON N G, et al. Off-vertical rotation produces conditioned taste aversion and suppressed drinking in mice[J]. Aviat Space Environ Med,1984,55(7):632-635. [6] LI Z Y, ZHANG X D, ZHENG J M, et al. Pica behavior induced by body rotation in mice[J]. ORL,2008,70(3):162-167. doi: 10.1159/000124289 [7] OSSENKOPP K P, MACRAE L K, BETTIN M A, et al. Body-rotation induced analgesia in male mice: effects of duration and type of rotation procedure[J]. Brain Res Bull,1988,21(6):967-972. doi: 10.1016/0361-9230(88)90036-6 [8] OSSENKOPP K P, BETTIN M A, KAVALIERS M. The effects of naloxone on body rotation-induced analgesia and anorexia in male mice[J]. Pharmacol Biochem Behav,1989,34(2):317-320. doi: 10.1016/0091-3057(89)90318-3 [9] 徐叔云, 卞如濂, 陈修. 药理实验方法学[M]. 3版. 北京: 人民卫生出版社, 2002: 882-887. [10] KIM M S, KIM J H, JIN Y Z, et al. Temporal changes of cFos-like protein expression in medial vestibular nuclei following arsanilate-induced unilateral labyrinthectomy in rats[J]. Neurosci Lett,2002,319(1):9-12. doi: 10.1016/S0304-3940(01)02422-3 [11] OSSENKOPP K P, PARKER L A, LIMEBEER C L, et al. Vestibular lesions selectively abolish body rotation-induced, but not lithium-induced, conditioned taste aversions (oral rejection responses) in rats[J]. Behav Neurosci,2003,117(1):105-112. doi: 10.1037/0735-7044.117.1.105 [12] KHAN Z, CAREY J, PARK H J, et al. Abnormal motor behavior and vestibular dysfunction in the stargazer mouse mutant[J]. Neuroscience,2004,127(3):785-796. doi: 10.1016/j.neuroscience.2004.05.052 [13] WU W J, SHA S H, MCLAREN J D, et al. Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat[J]. Hear Res,2001,158(1-2):165-178. doi: 10.1016/S0378-5955(01)00303-3 期刊类型引用(8)
1. 林琳,樊刚,李青,朱文静,薛嘉虹,魏瑾. 白藜芦醇对实验性自身免疫性心肌炎大鼠心肌巨噬细胞极化及心肌炎性损伤的影响. 陕西医学杂志. 2025(01): 27-32 .  百度学术
百度学术2. 陈倩倩,刘红静,韩洁. 通络消肿汤合温针灸联合西药治疗乳腺癌术后上肢淋巴水肿41例. 中医研究. 2023(03): 41-45 .  百度学术
百度学术3. 王玉寒,李曰,丁爽. 骨髓微环境中巨噬细胞调控造血机制的研究进展. 中国实验血液学杂志. 2023(04): 1242-1246 .  百度学术
百度学术4. 黄念,薛晓鸥,刘小丽,谢伟. 国家中药复方专利治疗异常子宫出血的治法和用药规律分析. 世界中西医结合杂志. 2023(09): 1705-1710 .  百度学术
百度学术5. 卡玉秀,刘维,丁久力,林芳芳,陈婌娟,顾庆香,岳青云,樊一桦. 生物碱类天然药物抗痛风作用及作用机制的研究进展. 天津中医药. 2023(12): 1621-1626 .  百度学术
百度学术6. 吕冰峰,秦锦龙. miR20a自噬炎症小体在子宫内膜异位组织中表达及意义. 中国计划生育学杂志. 2022(01): 139-142 .  百度学术
百度学术7. 赵佳敏,李茜如,高飞菲,巩志国,顾柏臣,白云洁,刘博. NLRP3在介导小鼠皮肤伤口修复过程中的作用. 中国兽医学报. 2022(04): 733-739 .  百度学术
百度学术8. 朱君,付立霞,支青,刘雄利,陈琳,田民义,王慧娟. 桑枝乙醇提取物通过NF-κB和MAPKs信号通路对LPS诱导RAW264.7细胞的抗炎作用. 中成药. 2022(11): 3648-3653 .  百度学术
百度学术其他类型引用(3)
-





 下载:
下载: