-
右美托咪定是一种α-2肾上腺素受体激动剂,由于它对认知功能、血流动力学稳定性和呼吸的影响较小,已被广泛用作麻醉辅助剂用于抗焦虑、镇静、术后镇痛等[1-2]。在临床上,我们发现达到相同镇静效果,梗阻性黄疸患者使用右美托咪定的用量低于非黄疸者,故猜测梗阻性黄疸患者使用该药有可能影响其药动学特征。目前,在婴儿、儿童、成人以及在肥胖和低蛋白血症人群中已有右美托咪定的药动学报道[3-6],而对梗阻性黄疸患者相关研究甚少。因此,本研究通过测定患者体内药物浓度,探讨梗阻性黄疸对右美托咪定药动学的影响。
目前已有多种分析方法用于测定血浆中的右美托咪定,包括高效液相色谱法、气相色谱-串联质谱法、高效液相色谱-串联质谱法[7-9],但这些方法存在分析时间长、灵敏度低、回收率低等缺点。本研究建立一种快速、灵敏、稳定的超高效液相色谱-串联质谱法测定人血浆右美托咪定的浓度,为右美托咪定药动学研究提供一种可靠的分析方法。
HTML
-
Agilent 1290 InfinityⅡ液相色谱仪、Agilent 6470三重四级杆质谱仪(Agilent Technologies,美国);真空浓缩旋转仪、低温高速离心机(Thermo,德国);XW 80A型涡旋混合器(医大仪器,上海);Mettler AE240十万分之一电子天平(梅特勒-托利多,瑞士);Millipore-Q超纯去离子水净化仪(Millipore,美国)。
-
右美托咪定对照品(批号:100523-201301)、卡马西平对照品(批号:100142-201706)(中国食品药品检定研究院);甲醇(色谱纯,美国Merck公司);甲酸(色谱纯,SIGMA公司);乙酸乙酯(分析纯,江苏强盛功能化学股份有限公司)。
1.1. 仪器
1.2. 试药
-
色谱柱为Agilent Eclipse Plus C18 RRHD column (2.1 mm×50 mm, 1.8 μm),甲醇-0.1%甲酸水 (75∶25)为流动相,流速0.2 ml/min,柱温:35 ℃,进样量2 μl,运行时间1.5 min。
-
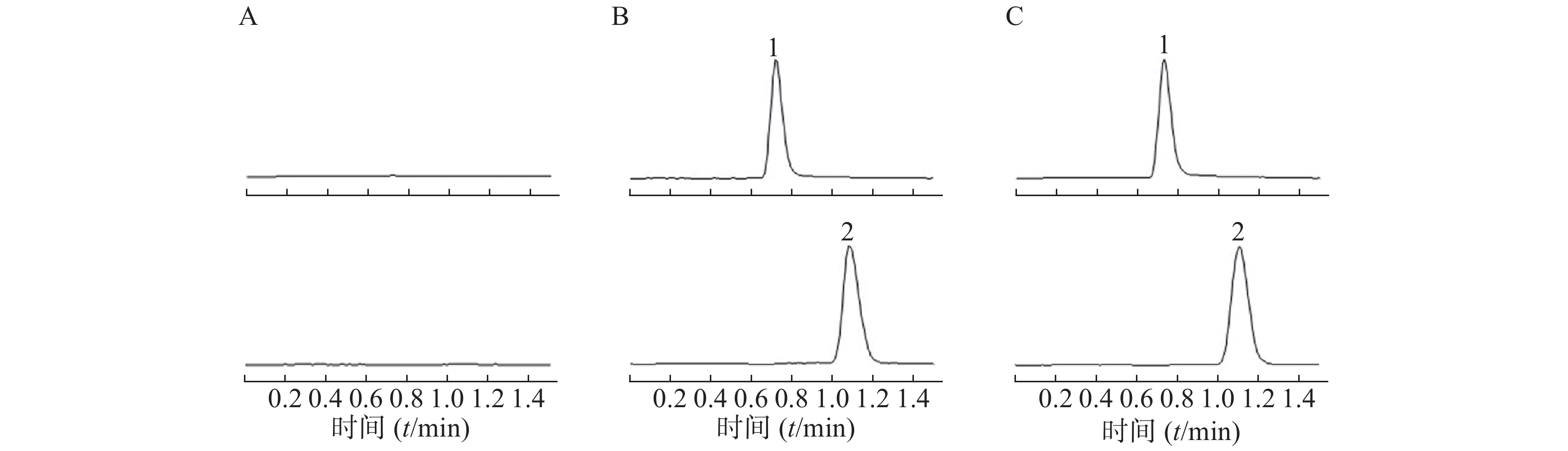
采用AJS ESI正离子模式,多反应离子监测模式(MRM)进行二级扫描;动态反应监测的离子对参数:右美托咪定201→95;卡马西平237→194。离子源参数设置:干燥气温度350 ℃;干燥气流速10 L/min;雾化器压力40 psi;鞘气温度400 ℃;鞘气流速11 L/min;毛细管电压4000 V;喷嘴电压500 V。右美托咪定保留时间为0.9 min,内标保留时间为1.1 min,色谱图如图1所示。
-
精密称取右美托咪定对照品10.00 mg,置于10 ml量瓶中,加甲醇溶解并稀释至刻度,摇匀,得质量浓度为1.00 mg/ml对照品储备液。再采用逐级稀释法配置质量浓度为0.01、0.05、0.1、0.5、1.00、5.00、10.00 ng/ml的系列对照品血浆溶液;随行质控样品中右美托咪定的低、中、高质量浓度分别为0.05、0.50、5.00 ng/ml。以上溶液量于4 ℃冰箱备用。
-
取200 μl血浆样品于离心管中,加入20 μl内标溶液、20 μl NaOH (1 mol/L)溶液和1 ml乙酸乙酯-氯甲烷(4∶1, V/V)提取溶剂,涡旋60 s,4 ℃条件下3000×g离心10 min,取上清液800 μl加入离心管中,真空浓缩干燥,加入100 μl流动相溶液,涡旋30 s使残渣溶解,4 ℃条件下3000×g离心10 min,取上清液进样。
-
按“2.3”和“2.4”项中的方法制备标准曲线,平行操作5份,按上述UPLC-MS/MS条件,连续进样分析,以对照品浓度 (X)为横坐标,右美托咪定的峰面积与内标的峰面积比值 (Y)为纵坐标,进行线性回归,得线性方程:Y=1.307 2X−0.015 6, r=0.999 8(r=0.999 9),线性范围为0.01~10.00 ng/ml,以信噪比为5时(S/N=5)测得右美托咪定的最低定量限为10 pg/ml,以信噪比为3时(S/N=3)测得右美托咪定的最低检测限为5 pg/ml。
-
按“2.3”和“2.4”项中的方法制备低、中、高3种浓度的质控样品,平行操作5份,连续3 d,计算实测浓度。如表1所示,精密度用相对标准偏差(RSD%)表示,结果日内精密度RSD%≤5.9%、日间精密度RSD%≤5.8%;准确度以相对回收率表示,实测浓度与理论加入浓度的比值即为相对回收率。结果表明日内、日间结果准确度范围在91.2%~105.6%。
浓度(ng/ml) 日内 日间 实测浓度(pg/ml) 精密度(%) 准确度(%) 实测浓度(pg/ml) 精密度(%) 准确度(%) 0.05 52.78±3.12 5.9 105.6 51.13±2.97 5.8 102.2 0.50 455.84±13.30 2.9 91.2 455.82±10.04 2.2 91.2 5.00 4939.46±33.17 0.7 98.8 4970.33±144.57 2.9 99.4 -
按“2.3”和“2.4”项中的方法制备低、中、高3种浓度含药血浆,平行操作5份,其待测物的峰面积作为Set 3;取空白人血浆按“2.4”项下处理,甲醇代替内标溶液,真空浓缩干燥后加入相应浓度对照品溶液使残渣溶解,使之浓度与Set 3的待测理论浓度一致,分别制备5份,其峰面积作为Set 2;将待测化合物标准溶液用甲醇稀释,使之与Set 3待测理论浓度一致,进样5次,其峰面积作为Set 1。基质效应为Set 2/Set 1,提取回收率计算公式为Set 3/Set 2,考察内标的基质效应和提取回收率操作步骤同上。低、中、高3种浓度待测物及内标提取回收率均在85.5%~93.1%之间,基质效应均在91.2%~95.6%之间,具体结果见表2。
待测物 浓度(ng/ml) 提取回收率(%) 基质效应(%) 右美托咪定 0.05 87.7±s7.1 95.6±6.1 0.50 90.5±5.4 94.3±8.3 5.00 93.1±4.2 91.2±7.9 内标 10.00 85.5±6.8 94.5±6.6 -
按“2.3”和“2.4”项中的方法制备低、中、高3种浓度含药血浆,分别考察样品室温放置6 h后处理,样品前处理后室温放置24 h,3次冻融循环以及−80 ℃保存30 d的稳定性,按“1.9”项操作测定样品浓度,计平均值,并计算RSD%及相对偏差RE%,测定结果的RE范围为95.4%~103.5%,RSD均小于15%,表明样本稳定性良好。
-
选取拟进行胆管手术的患者19名。根据术前血清总胆红素水平,分为黄疸组(n=11,平均年龄60.5岁,体重60.1 kg,身高159.8 cm)和对照组(n=8, 平均年龄60.5岁,体重60.1 kg,身高159.8 cm)。术前静脉输注右美托咪定(剂量为1.0 μg/kg),给药后使用含EDTA的采血管收集患者静脉血,采血时间点为0、0.5、1、2、3、5、10、12、15、20、30、40、60、90、120、180、240、300 min。血样经低温离心后,取其上清液于−80 ℃冰箱保存。待收集所有患者血浆后,使用本研究建立的UPLC-MS/MS法测定样品中右美托咪定的浓度。通过WinNonlin 7.0软件,非房室模型计算药动学参数,绘制平均血药浓度-时间曲线,如图2所示。使用SPSS 13.0软件,t-test分析比较两组数据的差异,结果见表3,与非黄疸组相比,梗阻性黄疸患者体内右美托咪定药动学参数cmax增加63.4%(P<0.01),AUC(0−t)、AUC (0−∞)和Vz分别增加78.9%、66.4%、82.5%(P<0.005),CLz降低42.1%(P<0.05)。结果显示,梗阻性黄疸患者体内右美托咪定消除减慢。
参数 对照组 阻塞性黄疸组 P值 cmax (ng/ml) 2.43±0.39 3.97±1.00 <0.01 tmax (t/min) 8.13±2.59 7.91±3.02 NS t1/2 (t/min) 146.72±82.28 135.10±49.92 NS CLz/F (ml∙min−1∙kg−1) 10.20±2.34 6.10±1.05 <0.001 AUC(0-t) (ng∙min∙ml−1) 77.70±15.15 139.16±40.59 <0.005 AUC(0-∞) (ng∙min∙ml−1) 101.94±19.68 169.58±36.62 <0.001 AUMC(0-∞)(ng∙min2∙ml−1) 19 068.07±12619.6 27 436.85±10 299.48 NS Vz(ml/kg) 2 072.09±904.67 1 199.87±454.79 <0.05 MRT(0-∞) (t/min) 172.4±86.81 159.96±60.01 NS 注:cmax:血峰浓度,tmax:达峰时间,t1/2:半衰期,CLz/F:清除率,AUC(0-t):药时曲线面积(0-t),AUC(0-∞):药时曲线面积(0-∞),AUMC(0-∞):一阶药时曲线面积(0-∞),Vz:表观分布容积,MRT(0-∞):平均驻留时间。
2.1. 色谱条件
2.2. 质谱条件
2.3. 标准曲线溶液的配制和质控样品的制备
2.4. 血浆样品前处理方法
2.5. 线性关系考察
2.6. 精密度与准确度试验
2.7. 基质效应和提取回收率
2.8. 稳定性试验
2.9. 右美托咪定药动学研究
-
本文所建立的UPLC-MS/MS法快速、灵敏、稳定,能应用于右美托咪定血浆样品的高通量分析。右美托咪定主要在肝脏发生生物转化[10],梗阻性黄疸可引起一定程度的肝功能异常(如药物代谢酶系统改变、胆管排泄功能受损、低蛋白血症)。根据总胆红素水平,将入组患者分为黄疸组(总胆红素水平>17.1 μmol/L)和非黄疸组(总胆红素水平<17.1 μmol/L)。本试验发现黄疸组患者右美托咪定的cmax、AUC(0−t)、AUC (0−∞)和Vz显著升高,CLz显著降低。结果提示,黄疸组与非黄疸组患者对右美托咪定药物处置存在差异,梗阻性黄疸可能会降低右美托咪定在患者体内的消除速度。该试验为右旋美托咪定在梗阻性黄疸患者中的临床使用剂量提供了一定的参考依据。










 DownLoad:
DownLoad: