-
奥卡西平(oxcarbazepine, OXC)是第二代抗癫痫药物,可用于儿童全面强直-阵挛发作,部分伴或不伴继发性全面发作的一线治疗[1],其具有与传统的抗癫痫药物(AEDs)如苯妥英钠、卡马西平和丙戊酸钠相同的疗效,但其对肝药酶和自身的诱导作用小,药物间相互作用较少,临床上可用于替代传统的AEDs。OXC是卡马西平(carbamazepine, CBZ)的一种10-酮类衍生物,但两者之间的药动学存在差异,OXC的耐受性好且不良反应少[2]。OXC是无活性的前体药物,在体内经过肝脏内细胞溶质芳基酮还原酶的作用转化为具有药理活性的中间代谢产物单羟基卡马西平(monohydroxy carbamazepine, MHD)[3-4]。国际抗癫痫联盟推荐MHD血清浓度的参考范围为3~35 μg/ml[5],有研究表明,当血药浓度高于30 μg/ml时,容易发生药物不良反应,且在许多患者中,不良反应间歇性的发生与MHD浓度的波动有关[6]。在临床用药中也发现OXC服药后的药物浓度个体化差异大,年龄、性别、体重、肝肾功能等均会影响MHD的药动学参数[7],故需要对其血药浓度进行监测。本研究在参考既往研究的基础上[8-12],对色谱条件进行了优化,并简化了血样处理的过程,建立了HPLC法测定OXC活性代谢产物MHD血药浓度的方法,该方法快速、简单、准确、选择性好、灵敏度高,为临床监测血药浓度、调整给药剂量提供了手段。
HTML
-
Agilent 1200高效液相色谱仪(美国Agilent公司);H1850R型台式高速冷冻离心机(湖南湘仪实验室仪器开发有限公司);DHG-9145A型电热恒温鼓风干燥箱(上海恒科仪器有限公司);SHB-B95A型循环水式多用真空泵(郑州长城科工贸有限公司);Discovery DV215CD型分析天平(美国OHAUS公司);Vortex-5型涡旋混合器(江苏海门市其林贝尔仪器制造有限公司)。
-
MHD对照品(美国CATO公司);内标:奥硝唑(中国食品药品检定研究院);甲醇、乙腈(色谱纯,上海科丰化学试剂有限公司);空白血浆(医院血库提供);超纯水(实验室自制)。
1.1. 仪器
1.2. 材料
-
色谱柱:ZORBAX Eclipse XDB-C18(150 mm×4.6 mm,5 μm),预柱为Eclipse XDB-C18(4.6 mm×12.5 mm,5 μm);流动相:水-乙腈(80:20,V/V);流速:1.0 ml/min;柱温:35 ℃;进样量:10 μl;双波长检测:在192 nm处检测MHD,318 nm处检测奥硝唑。
-
精密称取MHD对照品10 mg于10 ml的量瓶中,用甲醇溶解配制成浓度为1 mg/ml的储备液,置于−20 ℃下保存。
-
精密称取奥硝唑1.4 mg于10 ml的量瓶中,用甲醇溶解配制成浓度为140 μg/ml的内标工作液,置于−20 ℃下保存。
-
分别精密量取适量储备液,用甲醇稀释成20、50、100、200、300、400、500 μg/ml浓度梯度的标准对照溶液。取以上6个标准对照溶液,加入适量空白血浆,得2、5、10、20、30、40、50 μg/ml系列浓度的血浆标准曲线样品。同法配制相应的低、中、高浓度的血浆质控样品(QC),使得MHD对应的浓度分别为5、15、40 μg/ml。
-
取200 μl血浆样品,加入200 μl内标工作液、400 μl甲醇,涡旋混合30 s,10 ℃条件下13 000 r/min离心10 min,取上清液直接进样。
2.1. 色谱条件
2.2. 溶液及血浆样品的配制
2.2.1. 储备液的配制
2.2.2. 内标工作液的配制
2.2.3. 血浆标准曲线和质控样品的配制
2.3. 血浆样品的预处理
-
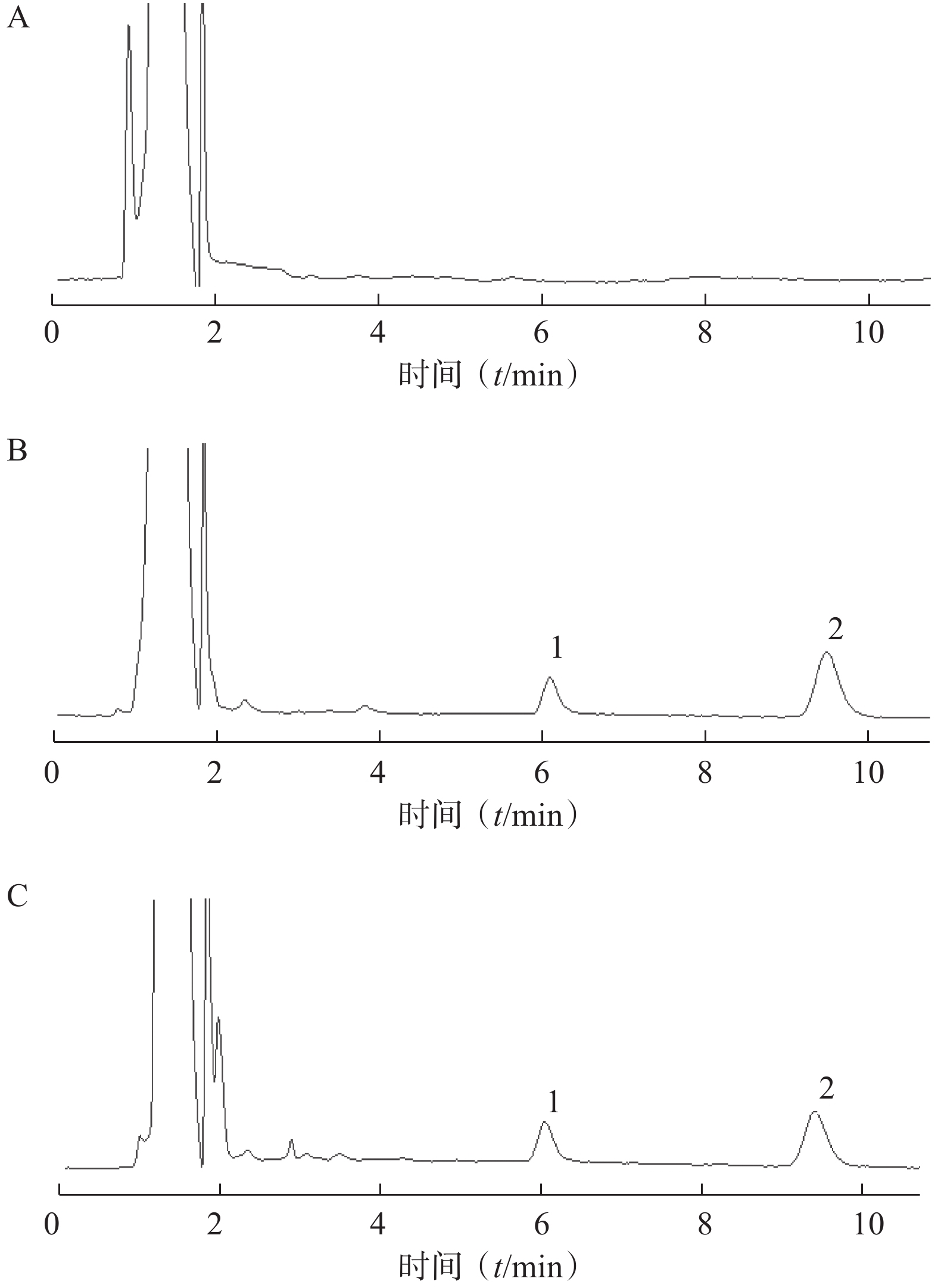
通过考察6份不同生物来源的空白血浆样品色谱图、空白血浆样品加入MHD对照品和内标的色谱图,以及临床实际用药后的患者血浆样品色谱图,以此反映方法的专属性。由图1可见,在本实验条件下,被测物MHD与内标的色谱峰分离良好,且血浆中的内源性物质不干扰测定。MHD和内标的保留时间分别为9.5 min和6.1 min。
-
取上述浓度为2、5、10、20、30、40、50 μg/ml的血浆标准曲线样品按“2.3”项下方法处理。经HPLC法分析,以测得OXC的峰面积与内标峰面积之比(Y)作为纵坐标,以血浆MHD浓度(X)为横坐标,得到回归方程为:Y=0.047 1X+0.022 2(r=0.998 6)。线性范围:根据标准曲线,MHD血药浓度在2~50 μg/ml范围内线性关系良好,其定量下限浓度为2 μg/ml。
-
配制定量下限、低、中、高(2、5、15、40 μg/ml)4种浓度的QC样品各6个,按“2.3”项下方法处理后测定,连续测定3 d,以当天的标准曲线计算QC样品的测定浓度,计算日内、日间精密度以及准确度。经测定,MHD的日内、日间精密度RSD均小于15%,准确度在95.57%~100.59%之间,均符合生物样品的测定要求,结果见表1。
理论浓度
(μg/ml)实测浓度
(μg/ml)RSD(%) RE(%) 日内精密度 日间精密度 2 1.85±0.16 8.64 12.21 −3.63 5 4.93±0.24 4.86 7.68 −4.43 15 14.32±0.37 6.86 6.16 −3.11 40 41.80±1.26 3.03 5.33 0.59 -
配制低、中、高(5、15、40 μg/ml)3种浓度的QC样品各6个,按“2.3”项下方法处理。同时另取18份空白血浆,除了不加系列对照品溶液和内标外,按“2.3”项下方法处理,在获得的上清液中加入MHD和内标溶液至相应浓度。进样分析,以每一浓度中2种不同处理方法的峰面积比值计算提取回收率。经测定,本法中MHD的平均提取回收率在89.62%~95.32%之间;内标的平均提取回收率为98.76%,符合生物学样品的分析要求,结果见表2。
化合物 浓度(μg/ml) 提取回收率(%) MHD 5 89.62±4.82 15 94.67±6.76 40 95.32±4.90 内标 140 98.76±5.92 -
取低、中、高(5、15、40 μg/ml)3种浓度的QC样品各5份,测定即时血药浓度,并在室温条件下放置4 h和10 h后测定样品血药浓度,求得RSD和RE值。经测定MHD血浆样品在室温下放置10 h后仍稳定,RSD均小于4.47%,RE值在1.50%~2.98%之间,结果见表3。
储存条件 理论浓度(μg/ml) 实测浓度(μg/ml) RSD(%) RE(%) 室温10 h 5 5.15±0.19 3.60 2.98 15 15.39±0.69 4.47 2.62 40 40.60±0.40 0.98 1.50 冻融3次 5 5.47±0.15 2.83 9.41 15 15.79±0.32 2.06 5.24 40 40.75±1.10 2.71 1.86 处理后36 h 5 5.39±0.27 5.09 7.74 15 15.66±0.58 3.70 4.39 40 39.46±1.80 4.57 −1.34 −20 ℃,30 d 5 5.16±0.23 4.39 3.19 15 14.62±0.39 2.64 −2.53 40 40.37±0.58 1.44 0.93 -
取低、中、高(5、15、40 μg/ml)3种浓度的QC样品各5份,测定即时血药浓度,并于−20 ℃冰箱中冷冻保存24 h后室温下解冻1 h后测定,反复冻融3次,求得RSD和RE值。经测定MHD血浆样品反复冻融3次后仍能保持稳定,RSD均小于2.83%,RE值在1.86%~9.41%之间,结果见表3。
-
取低、中、高3个浓度水平的血浆QC样品(5、15、40 μg/ml)各5份,测定即时血药浓度,然后放置在自动进样器内12 h、36 h后再次测定,求得RSD和RE值。经测定处理后的MHD血浆样品在进样器内放置36 h仍能保持稳定,RSD均小于5.09%,RE值在−1.34%~7.74%之间,结果见表3。
-
取低、中、高3个浓度水平的血浆质控样品(5、15、40 μg/ml)各5份,测定即时血药浓度,并置于−20 ℃冰箱中冻存30 d后取出解冻后测定,求得RSD和RE值。经测定MHD血浆样品在−20 ℃冰箱中冻存30 d后仍能保持稳定,RSD均小于4.39%,RE值在−2.53%~3.19%之间,结果见表3。
3.1. 专属性试验
3.2. 标准曲线与线性范围
3.3. 日内、日间精密度和准确度
3.4. 提取回收率
3.5. 稳定性试验
3.5.1. 室温稳定性试验
3.5.2. 冻融稳定性试验
3.5.3. 处理后的血浆样品在自动进样器中储存的稳定性试验
3.5.4. 长期稳定性试验
-
目前,有关MHD的检测方法的文献很多,其中,高效液相色谱法和高效液相色谱-质谱联用法都有报道,后者虽有灵敏度高、专属性强的特点,但其仪器昂贵,且需专业人员进行操作,很难在大部分的医疗机构普及[13-15]。另外,国内外文献报道中多使用固液萃取法或液液萃取法进行血样的前处理,但这种处理过程相对复杂、耗时,且经济成本较高[11, 16]。本方法采用甲醇沉淀蛋白进行血样的前处理,建立了HPLC法测定人血浆中MHD血药浓度的方法,整个过程操作简单、快速,样品分析时间较短,适用于临床大量样品的连续检测。
文献报道的流动相有乙腈-10 mmol/L磷酸二氢钾溶液(33∶67,V/V)[12]、水-乙腈(65∶35,V/V)[17]、水-甲醇-乙腈(64∶30∶6,V/V/V)[18]等。本方法采用了水-乙腈,按不同的配比进行试验,发现当水-乙腈的比例为80∶20时,色谱峰的峰形、出峰时间及分离度最佳。文献采用卡马西平[17]、苯巴比妥[19]和奥硝唑[20]等作为内标,本研究通过筛选发现奥硝唑的保留时间为6.1 min,不仅与MHD的保留时间相近,又能与其有很好的分离,且其性质稳定,满足内标的要求。
本实验建立的测定人血浆中OXC活性代谢产物MHD的HPLC法,MHD线性回归方程中的r=0.998 6,说明血药浓度在2~50 μg/ml范围内具有良好的线性关系,日内、日间精密度RSD均小于15%,准确度在95.57%~100.59%之间,MHD及内标的平均提取回收率在89.62%~98.76%之间。血浆样品的稳定性试验证明,在室温放置10 h、反复冻融、处理后放置进样器36 h以及低温保存30 d的情况下,样品未见明显降解,仍能保持稳定。本研究建立的HPLC法操作快速简单,精密度、回收率高,稳定性好,专属性强,不受血浆中内源性物质的干扰,结果准确可靠,且灵敏度高,适用于奥卡西平临床血药浓度的监测。
目前癫痫治疗主要以药物治疗为主,奥卡西平是第二代抗癫痫药物,我国诸多癫痫病专家也建议将其作为癫痫部分性发作和全面强直阵挛发作的首选药物[21]。但奥卡西平使用过程中可能出现瘙痒、荨麻疹、血管性水肿等超敏反应,包括Stevens Johnson综合征中毒性表皮坏死松解症[22]等,还可引起低钠血症、头晕、胃肠道不适等不良反应,有文献报道,其疗效及不良反应可能与血药浓度密切相关[23],因此开展奥卡西平血药浓度的测定,能提高药物治疗的疗效,同时可以有效避免或减少可能产生的药物不良反应,提高癫痫患者服药的依从性。本研究建立了测定人血浆中MHD血药浓度的方法,应用于临床,为临床个体化给药提供依据,值得临床推广使用。








 DownLoad:
DownLoad: