-
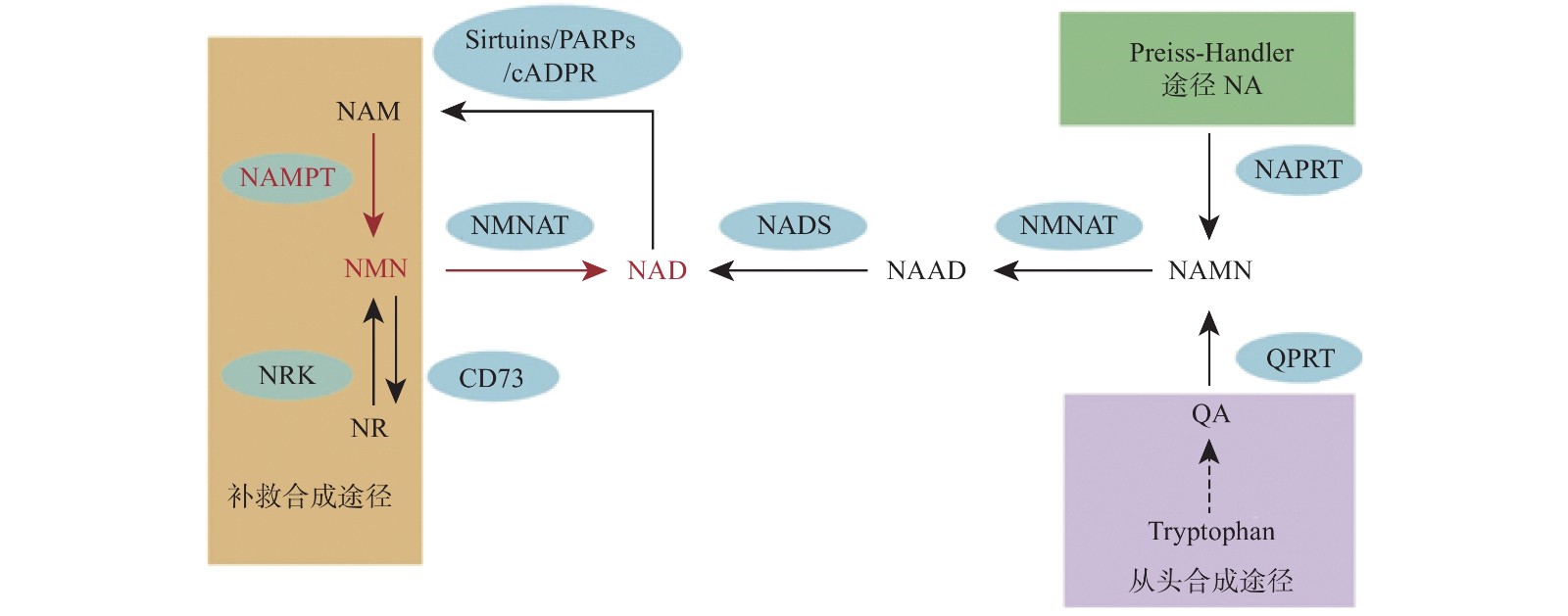
烟酰胺腺嘌呤二核苷酸(NAD)是真核细胞中的一种重要的代谢氧化还原辅酶,它在各种生物过程中起关键作用,包括新陈代谢、衰老、细胞死亡、DNA 修复和表达[1-2]。NAD主要有3种生物合成途径,在哺乳动物中,补救途径是NAD的主要来源并且该途径只有两个步骤(图1)。NAD 在该途径中的合成速率主要由烟酰胺磷酸核糖基转移酶(Nampt)决定,该步骤首先将烟酰胺(NAM)和5-磷酸核糖基-1-焦磷酸(PRPP)转化为烟酰胺单核苷酸(NMN)。然后,在第二步中将NMN与ATP偶联并转化为NAD[3]。直接使用NMN可以弥补NAD的不足,因此NMN可以参与多种疾病的调节,如2型糖尿病、肥胖症、心力衰竭、神经再生和炎症等[4-8]。
溃疡性结肠炎是炎症性肠病的一种。近年来,其病因和发病机制的研究受到广泛关注[9-11]。目前治疗溃疡性结肠炎的手段主要是对症治疗,如抗感染药、糖皮质激素和免疫抑制剂等,但这些药物长期使用会带来很多不良反应[12-13]。NAD与多种炎症性疾病有关,如败血症和类风湿性关节炎[14-15]。此外,有报道显示,炎症性肠病患者结肠组织和血清中Nampt浓度升高,并将Nampt作为小儿炎症性肠病严重程度的标志物[16-17]。因此,作为NAD合成的中间重要产物NMN,也有可能对炎症性肠病产生一定作用。与此同时,目前有很多含有NMN的保健品问世,但是由于NMN参与调节的功能非常复杂,并且有研究表明NMN可以促进肿瘤的增殖等作用[18],因此,补充NMN并不一定对人体都有益。并且,NMN作为保健品需要长期服用,但对于一些患有如炎症性肠病的慢性病患者,评价其对于患者的安全性极其重要。
本研究主要考察NMN对DSS诱导的溃疡性结肠炎小鼠模型的作用。以评价NMN作为保健品是否适用于患有溃疡性结肠炎的患者。
HTML
-
雄性C57小鼠(8周龄,上海西普尔-必凯实验动物有限公司),放于实验室适应2周后进行造模。按照之前的方法进行模型制备[19],将DSS(美国MP公司,分子量为36 000~50 000)溶于水中,至终浓度为3%,让小鼠自由饮用。
-
将NMN[音芙医药科技(上海)有限公司]溶于生理盐水中,制备不同浓度的NMN溶液,将雄性C57小鼠分为6组,分别为生理盐水对照组,3、10、30、100、300 mg/kg NMN的不同剂量组。按照容积0.1 ml/10 g,通过灌胃(ig)和腹腔注射(ip)两种方式,从第1天开始给药,每天1次,直至实验观察结束。
-
按照之前的评分标准进行评分[19],对体重下降程度、大便性状、血便情况分别进行评分,而后进行加和,计算总分数。具体评分标准见表1。
评分 体重下降(%) 大便性状 血便情况 0 无 正常 无 1 1~5 — — 2 5~10 半稀便 潜血 3 10~15 — — 4 ≥15 稀便 肉眼血便 注:“—”表示无此项评分。 -
小鼠处死后,取整个结肠部位,测量长度进行比较。而后将结肠下段部位组织用4%多聚甲醛固定24 h,然后包埋在石蜡中。准备厚度为4 μm的切片,按照之前的实验方法,进行HE染色[19]。
-
本实验指标均为定量数据,数据以(
$\bar x \pm s$ )表示。两两比较采用t-test法进行,采用GraphPad Prism 7统计软件进行统计分析。
1.1. DSS诱导肠炎模型的制备
1.2. 分组及给药情况
1.3. 疾病评分(DAI)
1.4. 形态学检测及组织学染色
1.5. 统计分析
-
为了确定实验的最佳观察时间,首先对小鼠的生存曲线进行测定。将100和300 mg/kg的NMN通过灌胃的给药方式,检测3%DSS诱导的溃疡性结肠炎模型小鼠生存时间(图2),在给药第9天时,300 mg/kg组中有2只小鼠死亡,生理盐水对照组和100 mg/kg组在第10 天出现小鼠死亡。第17天时,300 mg/kg组小鼠全部死亡,而在第20天时,生理盐水对照组和100 mg/kg组全部死亡。3组生存曲线之间并无统计学差异。由于在给药第9天时,有小鼠出现死亡,因此我们将后面实验的观察时间设定为8 d。
-
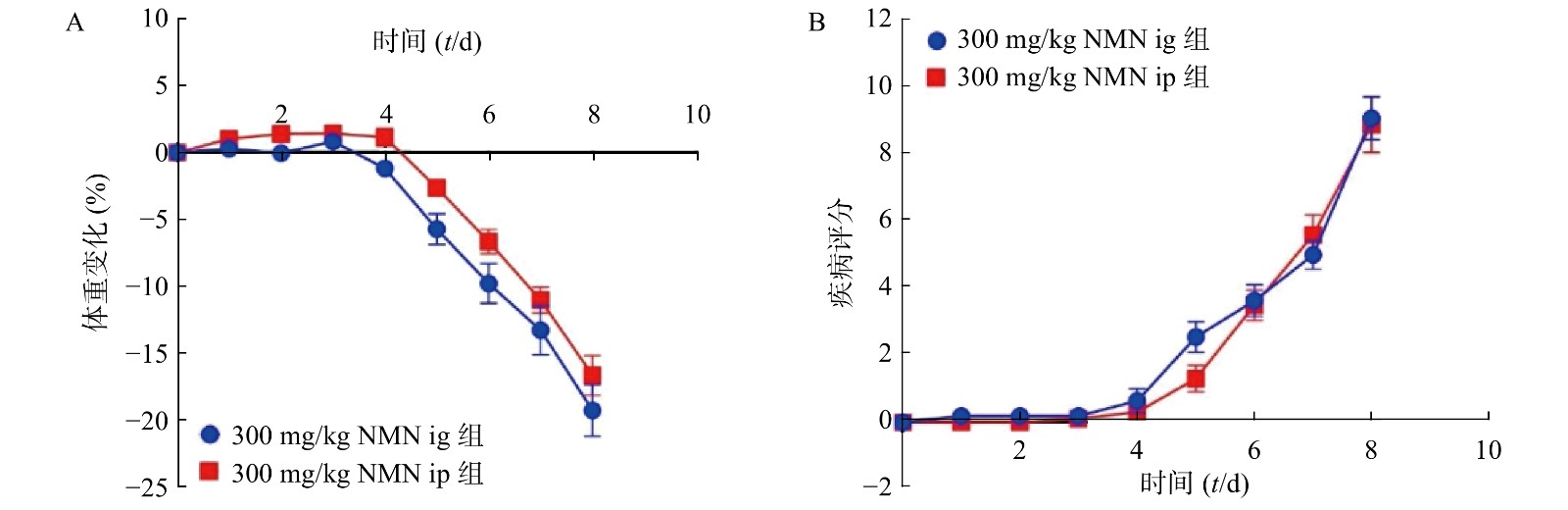
为了考察不同给药方式对小鼠的影响,我们分别通过灌胃和腹腔注射两种给药方式,观察NMN对于小鼠体重和DAI的影响,如图3所示,灌胃和腹腔注射两种给药方式对溃疡性结肠炎模型小鼠体重和DAI分数的影响无显著性差异,二者都在第5天时表现出体重下降并出现稀便情况。因此,为了更真实的模拟人用药环境,我们在后续实验中均采用灌胃的给药方式。
-
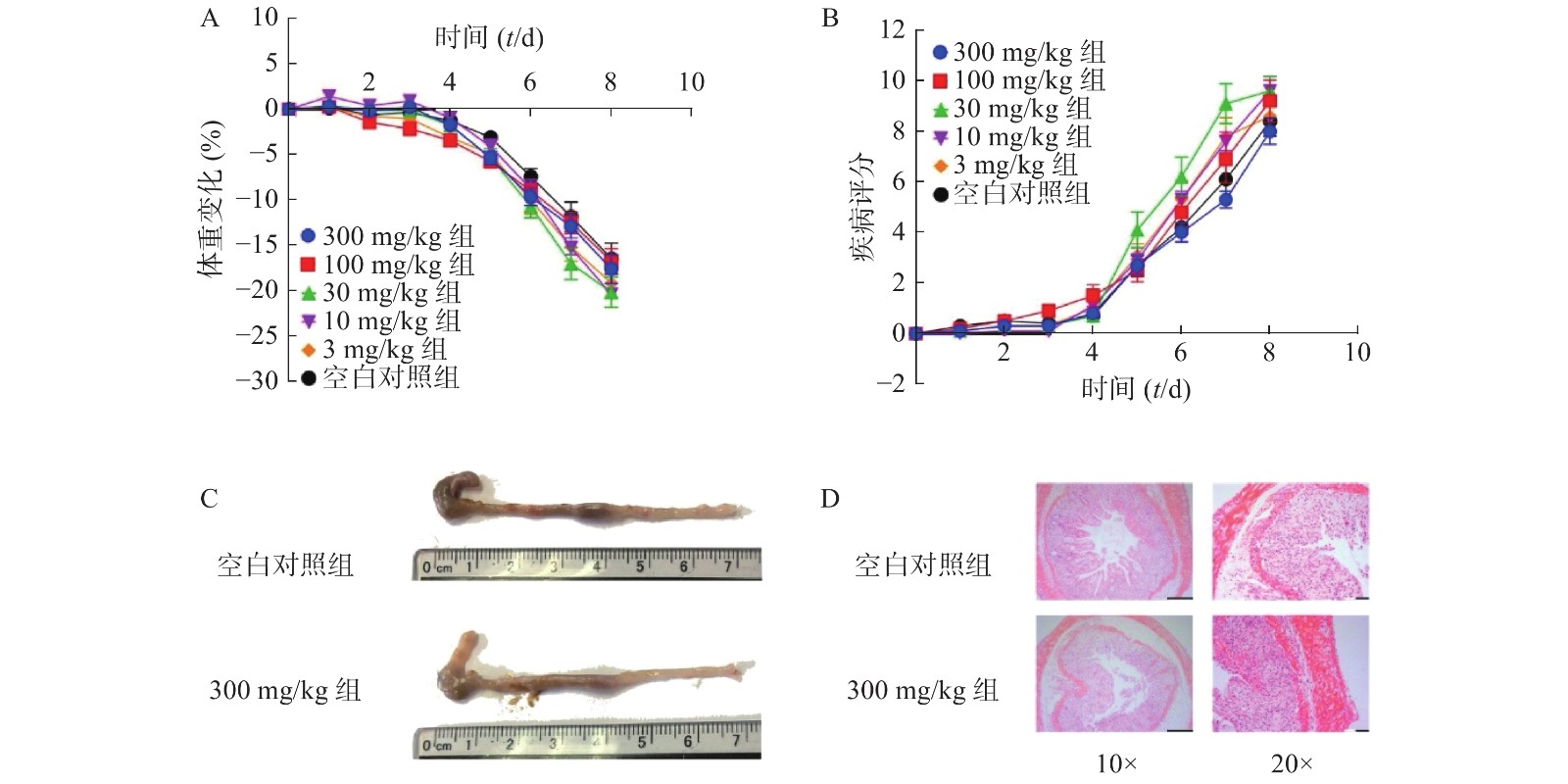
为了进一步考察不同浓度NMN对DSS诱导的溃疡性结肠炎模型小鼠的影响,我们应用了不同浓度的NMN通过灌胃给药的方式,分别对小鼠的体重和DAI进行了检测。结果显示(图4A、4B),不同浓度的NMN对小鼠的体重和DAI的影响,与对照组相比均无显著性差异。为了更进一步确认,我们又对结肠长度和结肠形态进行了检测,结果显示(图4C、4D),300 mg/kg的NMN与对照组相比,肠道长度和免疫组化切片结果并无显著性差异。
2.1. NMN对DSS诱导的溃疡性结肠炎模型小鼠生存时间的影响
2.2. NMN不同给药方式对DSS诱导的溃疡性结肠炎模型小鼠体重和DAI的影响
2.3. NMN对DSS诱导的溃疡性结肠炎模型小鼠结肠长度与形态的影响
-
本研究测定了不同剂量的NMN对DSS诱导的溃疡性结肠炎小鼠的体重、DAI评分、结肠长度和肠道形态的影响,即NMN对DSS诱导的溃疡性结肠炎小鼠并无显著作用。
NMN作为NAD的前体,其功能目前认为是通过NAD来体现的,NNM和NAD的代谢是紧密联系的。NAD在人体内有3条生化途径:Preiss-Handler途径、从头合成途径和补救合成途径(图1)[20]。Preiss-Handler途径从NA开始,经过NAPRT催化变成NAMN,经过NMNAT的催化,变成NAAD,然后再被NAD合成酶NADS催化成NAD[21]。从头合成途径首先是从食物中摄取的色氨酸开始,经过一系列体内生化反应生成喹啉酸(QA),而后经过(QPRT催化生成NAMN进入Preiss-Handler途径[22]。补救合成途径从NAM开始,然后经过NAMPT催化后,变成NMN,NMN同样通过NMNAT酶的催化转变成NAD,而后NAD在经过3个消耗途径NAD依赖的去乙酰化酶(Sirtuins)、多聚ADP核糖聚合酶(PARPs)、环腺苷二磷酸核糖(cADPR)合酶后变成烟酰胺完成循环[2]。NAD的含量在这3条途径下保持平衡,补救合成途径是人体NAD的主要来源,该过程中的NAMPT是这个循环的限制步骤[23]。
NMN作为NAD合成的重要中间产物,存在于多种类型的细胞中,并参与了不同的生物学过程。NMN可防止由NAD分解代谢酶而引起的NAD耗竭。目前报道的NMN大部分作用对人体都是有益的,但是随着研究的深入,NAD的增加也能够加重某些疾病的发展,如炎症和肿瘤[18, 24-25]。近期有研究表明,在克罗恩结肠炎和溃疡性结肠炎患者的结肠组织中,血浆Nampt和mRNA水平均显著升高[16]。更有研究表明,Nampt的特异性抑制剂FK866,改善了DSS诱导的溃疡性结肠炎模型小鼠症状,并抑制了小鼠中与炎症相关的肿瘤发生。由于Nampt是NMN的重要合成生物酶,因此,我们怀疑NMN可能也会加重DSS诱导的溃疡性结肠炎模型小鼠症状。基于此,我们首先检测了不同剂量的NMN对模型小鼠的生存曲线的影响,发现与对照组相比,NMN并不会显著改变模型小鼠的生存时间。而后我们发现不同给药方式也不能显著改变小鼠溃疡性结肠炎的状态。最后我们又检测不同浓度的NMN对于肠炎小鼠的影响,发现300 mg/kg 剂量以下的NMN,对于模型小鼠的体重、DAI评分、结肠组织均不能引起显著性改变。因此,NMN对DSS诱导的溃疡性结肠炎小鼠并无影响。
综上,NMN具有广泛的人体生物调节作用,目前市面上出现了很多含有NMN的保健品。虽然我们的研究结果表明NMN对DSS诱导的溃疡性结肠炎模型小鼠无明显毒副作用,但是对于NMN的副作用仍需继续考察,特别是对于长期使用NMN的人群来说,充分考察其安全性尤为重要。









 DownLoad:
DownLoad: