-
痛风在中医理论中属于“痹证”、“白虎历节”范畴[1]。中医辨证分型主要有湿热蕴结型、寒湿痹阻型、肝肾亏虚型、痰瘀痹阻型、瘀血阻滞型、脾虚湿盛型和寒热错杂型[2],在治疗上主要以泄浊利湿、通络止痛为主[3]。茵连痛风颗粒为上海中医药大学附属岳阳中西医结合医院自制制剂,是著名夏氏外科传人夏涵教授数十年的经典经验方。该制剂由茵陈、连钱草和伸筋草三味中药组成,用于治疗间歇期痛风性关节炎[4]。茵连痛风颗粒的成分多样,前期研究中发现其不同部位群对痛风模型大鼠均有不同程度的抗炎镇痛作用[5],但其作用有效成分和疾病靶标的关系尚不明确,抗炎镇痛作用可能与香豆素[6]、黄酮、有机酸[7]、三萜类、生物碱类以及蒽醌类[8]等成分有关。因此,笔者采用网络药理学方法对茵连痛风颗粒的抗炎镇痛作用机制进行分析,预测茵连痛风颗粒的主要活性成分和潜在靶标部位,以期为该制剂建立临床疗效的质量评估体系提供支持。
HTML
-
本研究利用中药系统药理学数据库和分析平台(TCMSP)[9](http://tcmspw.com/tcmsp.php)检索获得茵连痛风颗粒中三味中药的化学成分,通过TCMSP筛选出化学成分对应的数据,预测成分的口服利用度(OB) 和类药性(DL)[10],以OB≥30%且DL≥0.18作为筛选条件,筛选出活性成分,再将其依次输入TCMSP,查找得到潜在的靶点蛋白,并通过Uniprot数据库(https://www.uniprot.org/)[11]匹配蛋白所对应的基因。
-
将茵连痛风颗粒的活性成分及潜在靶点导入Cytoscape 3.7.2软件(http://www.cytoscape.org /),构建并预测茵连痛风颗粒的活性成分及靶点网络。
-
基于OMIM数据库[12]检索抗炎、镇痛相关靶蛋白基因,搜索关键词"pain"、"ache"、"soreness"、"inflammation"、"inflammatory",所有靶标均通过UniProt数据库获取其UniProt ID信息。
-
将OMIM中获取到的蛋白质信息导入STRNG数据库,筛选出蛋白相互作用分值>0.7的高置信数据[13],用Cytoscape 3.7.2构建与抗炎镇痛有关的蛋白质-蛋白质相互作用(PPI) 网络。
-
利用Cytoscape 3.7.2中的Merge功能,将茵连痛风颗粒活性成分-预测靶点网络与抗炎镇痛的PPI网络合并,确定两者共有的潜在作用靶点。通过Cytoscape3.7.2构建茵连痛风颗粒活性成分-潜在靶点网络,并分析茵连痛风颗粒抗炎镇痛的潜在靶点。
-
采用David v6.8数据库(https://david.ncifcrf.gov/home.jsp)[14-16],对与抗炎镇痛相关的茵连痛风颗粒活性成分-潜在靶点网络中的蛋白进行基于基因组百科全书(KEGG)的生物通路富集分析,并利用OmicShare对通路进行可视化,采用Davidv6.8数据库,对与抗炎镇痛相关的茵连痛风颗粒活性成分-潜在靶点网络中的蛋白进行基因本体GO功能富集分析[13]。
1.1. 茵连痛风颗粒活性成分及靶点的筛选
1.2. 茵连痛风颗粒活性成分-预测靶点网络的构建
1.3. 抗炎镇痛靶点的收集
1.4. 茵连痛风颗粒蛋白互作网络的构建
1.5. 网络合并
1.6. 通路及生物过程的富集分析
-
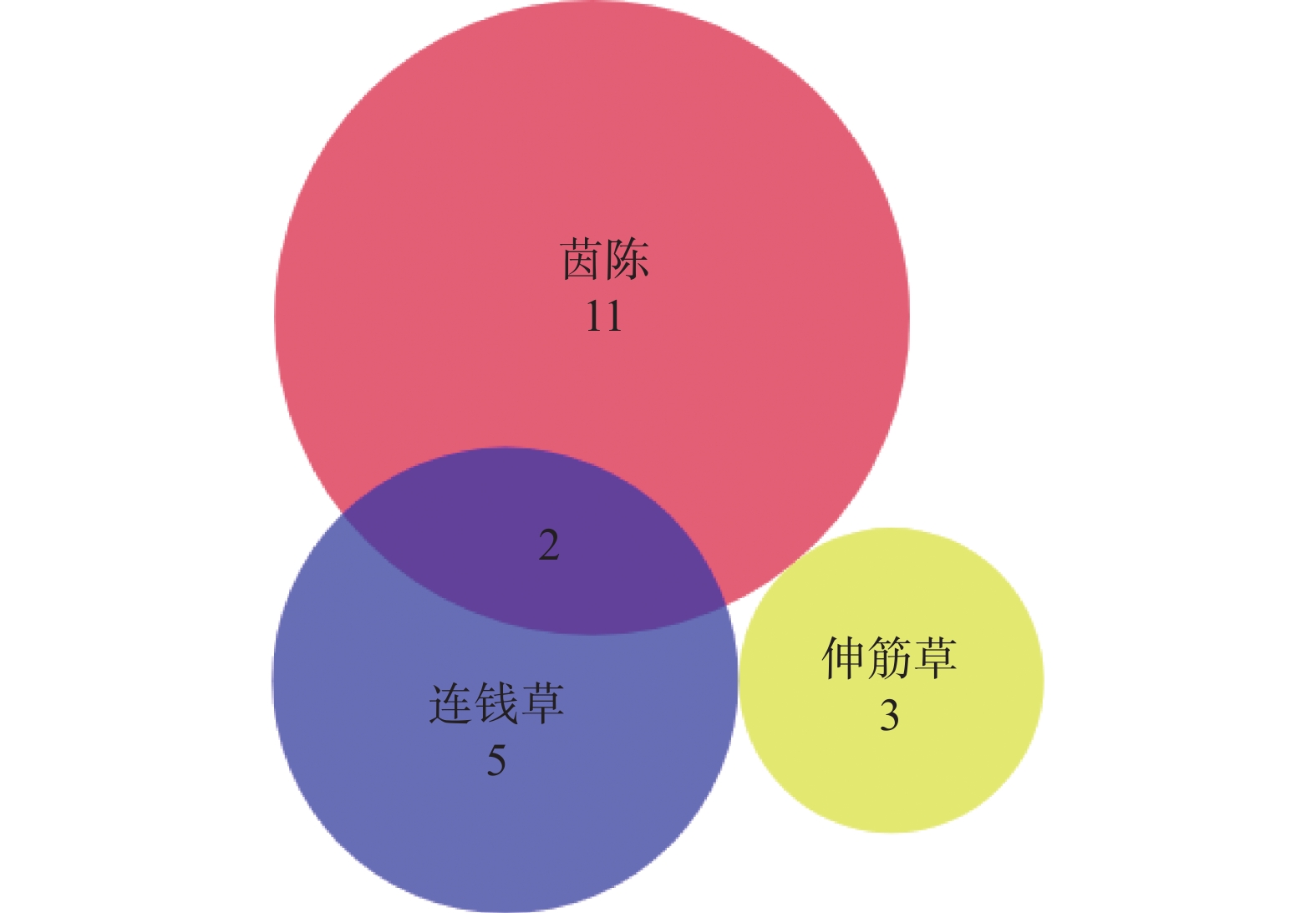
在TCMSP数据库中搜索“茵陈”、“连钱草”、“伸筋草”三味中药的化学成分,共得到175个化学成分,以OB≥30%且DL ≥0. 18作为筛选条件,删除无对应靶点的成分,共获得23个潜在有效成分(表1),其中,茵陈和连钱草共有成分为槲皮素和β-谷甾醇[17](图1)。
编号 活性成分 OB(%) DL 来源 YC1 异鼠李素 49.60 0.31 茵陈 A1 β-谷甾醇 36.91 0.75 茵陈 YC2 茵陈黄酮 48.96 0.41 茵陈 YC3 芫花素 37.13 0.24 茵陈 YC4 蓟黄素 30.35 0.30 茵陈 YC5 异茵陈黄酮 57.40 0.41 茵陈 YC6 紫杉醇 46.11 0.33 茵陈 YC7 依帕替丁 42.55 0.37 茵陈 YC8 茵陈色原酮 57.56 0.31 茵陈 YC9 4'-甲基茵陈色原酮 72.18 0.35 茵陈 YC10 脱甲氧基姜黄素 52.33 0.25 茵陈 YC11 茵陈黄酮A 68.32 0.24 茵陈 A2 槲皮素 46.43 0.28 茵陈 LQC1 金合欢素 34.97 0.24 连钱草 LQC2 蒙花苷 39.84 0.71 连钱草 LQC3 丁子香萜 55.38 0.78 连钱草 A1 β-谷甾醇 36.91 0.75 连钱草 LQC4 山奈酚 41.88 0.24 连钱草 LQC5 木犀草素 36.16 0.25 连钱草 A2 槲皮素 46.43 0.28 连钱草 SJC1 谷甾醇 36.91 0.75 伸筋草 SJC2 福莫尼汀 69.67 0.21 伸筋草 SJC3 豆甾醇 43.83 0.76 伸筋草 -
将筛选出的23个茵连痛风颗粒潜在有效成分导入到TCMSP,查找相应的靶点,得到686个潜在靶点蛋白,并利用Uniprot数据库匹配对应基因。将结果导入到Cytoscape 3.7.2软件中进行网络构建,得到茵连痛风颗粒活性成分靶点网络(图2)。该网络包含228个节点、686条边,红色六边形表示茵陈特有的活性成分、紫色六边形表示连钱草特有的活性成分、绿色六边形表示伸筋草特有活性成分、黄色箭头表示两个成分共有的活性成分、粉色菱形表示单味药的作用靶点、湖绿色菱形表示两味药共同的作用靶点、蓝色菱形表示三味药共同的作用靶点。如图2所示,23个茵连痛风颗粒的活性成分均具有多靶点,不同靶点可对应相同成分,不同的成分可对应相同的靶点。
-
在OMIM数据库检索与抗炎镇痛相关的基因,共检索到286个相关基因靶点。与茵陈活性成分共有32个共同靶基因,与连钱草活性成分共有35个共同靶基因,与伸筋草活性成分共有7个共同靶基因(图3)。
-
将茵连痛风颗粒预测所得的基因与抗炎镇痛相关基因进行映射后得到共有靶基因,输入STRNG数据库进行蛋白-蛋白相互作用分析。在分析过程中,选取物种为Homosapiens,并将蛋白-蛋白互作得分>0.7的基因输入Cytoscape 3.7.2进行网络可视化,共有37个节点,285个连线。应用软件中的NetworkAnalysis plugin对网络图中节点进行统计,分析其在图中的作用,自由度越大,该节点在网络中的生物功能则越多。同时,颜色越深代表该节点自由度较大、生物功能较多(图4)。进一步对与抗炎镇痛潜在靶点网络进行分析,网络中有17个靶点的自由度值大于平均度值15.41,预测可能为茵连痛风颗粒活性成分发挥抗炎镇痛疗效的潜在靶点。
-
利用David平台进行GO功能富集分析,得到了293个GO条目,根据P<0.05筛选出211个GO条目,针对前10条BP分析、CC分析、MF分析绘制直方图(图5)。其中,生物过程条目170条,主要包括炎症反应、基因表达的正调控、IL -6的正调控等;细胞组成条目16个,主要包括I-kappaB/NF-kappaB复合体、细胞质核周区域、GABA-A受体复合物等;分子功能条目25条,主要包括细胞因子活性、酶结合、蛋白质异二聚活性等。
利用David平台的KEGG通路富集分析功能,得到79条信号通路,根据P<0.001筛选出50条信号通路(图6);包括TNF信号通路、NOD受体信号通路、NAFLD、MAPK信号通路、Ras 信号通路、核转录因子B信号通路等。
-
基于上述信息,构建活性成分-靶点-通路图,以全面阐述茵连痛风颗粒抗炎镇痛的作用机制(图7); 图中有112个节点和462条边,绿色菱形代表茵连痛风颗粒活性成分,红色圆形代表潜在靶点,黄色箭头代表信号通路,边代表三者之间的相互作用,节点越大表示其抗炎镇痛的作用越大。
2.1. 茵连痛风颗粒活性成分筛选
2.2. 茵连痛风颗粒活性成分-靶点网络的构建
2.3. 抗炎镇痛靶点信息的收集和筛选
2.4. 茵连痛风颗粒抗炎镇痛靶标蛋白-蛋白相互作用分析及网络可视化构建
2.5. GO功能和KEGG通路的富集分析
2.6. 活性成分-靶点-通路图的构建
-
茵连痛风颗粒由茵陈、连钱草和伸筋草三味中药组成,茵陈具有清利湿热、利胆退黄的功效;连钱草具有利湿通淋,清热解毒,散瘀消肿的功效;伸筋草具有祛风除湿、舒筋活络的功效。三者合用增强了清热利湿,通络止痛的作用[18]。
本研究通过TCMSP、Uniprot数据库筛选潜在的抗炎镇痛活性成分,得出槲皮素、β-谷甾醇、茵陈色原酮、异鼠李素等23个黄酮类、甾体类成分。这些成分与现有文献报道中关于抗炎镇痛的活性成分相符[19-22]。其中,共有成分槲皮素为黄酮类化合物,有研究表明槲皮素对炎症反应具有良好的抑制作用,对化学刺激疼痛模型和热刺激疼痛模型均有显著的镇痛作用[23]。共有成分β-谷甾醇为甾体类化合物,具有类似于氢化可的松的抗炎活性,其抗炎机制主要是通过局部免疫调节来发挥抗炎作用[24]。
通过OMIM数据库、STRNG数据库及Cytoscape软件构建抗炎镇痛靶标蛋白-蛋白交互网络,揭示茵连痛风颗粒通过多靶点起到抗炎镇痛的作用。本研究共得到PTGS2、TNF、IL-6、IL-10等37个茵连痛风颗粒抗炎镇痛的关键靶点。前列腺素内过氧化物合酶PTGS),又称为环氧合酶(COX),存在两个亚型,即COX-1和COX-2[25]。PTGS2产生炎症性前列腺素,参与炎症的发生,同时与其他预测所得出的靶点都有密切联系[26-27]。TNF[28]、IL-6有致痛和痛觉增敏的作用,IL-10有活性较强的抗炎作用,除具抑制促炎细胞因子的产生外,还能促进其他抗炎因子的产生、下调促炎因子受体的表达[29]。PTGS2等靶点是抗炎镇痛的关键靶点,在炎症反应、基因表达等生物过程中起着至关重要的作用。
KEGG富集结果表明,茵连痛风颗粒抗炎镇痛关键靶点基因显著富集在TNF信号通路、MAPK信号通路、核转录因子B信号通路等。茵陈中的黄酮类成分具有重要的抗炎活性,其潜在的抗炎机制可能与其对NF-κB和MAPK信号通路的抑制有关[6]。研究表明[30],茵陈色原酮通过JNK途径激活转录因子E2相关因子2(Nrf2)及其下游靶标血红素加氧酶1(HO-1)水平,减弱LPS诱导的小胶质细胞炎症反应,也可有效抑制MyD88和Toll/IL-1接头蛋白(TIRAP)介导的信号通路,进而抑制LPS诱导的NF-κB、蛋白激酶B(Akt)和MAPK激活的炎症基因活化,从而发挥抗炎作用。
综上所述,本研究基于网络药理学,对茵连痛风颗粒抗炎镇痛的多成分、多靶点和多信号通路进行相关性研究,初步揭示了茵连痛风颗粒中三味中药的抗炎镇痛分子机制,为进一步开展和实验验证茵连痛风颗粒抗炎镇痛作用机制提供理论依据。














 DownLoad:
DownLoad: