-
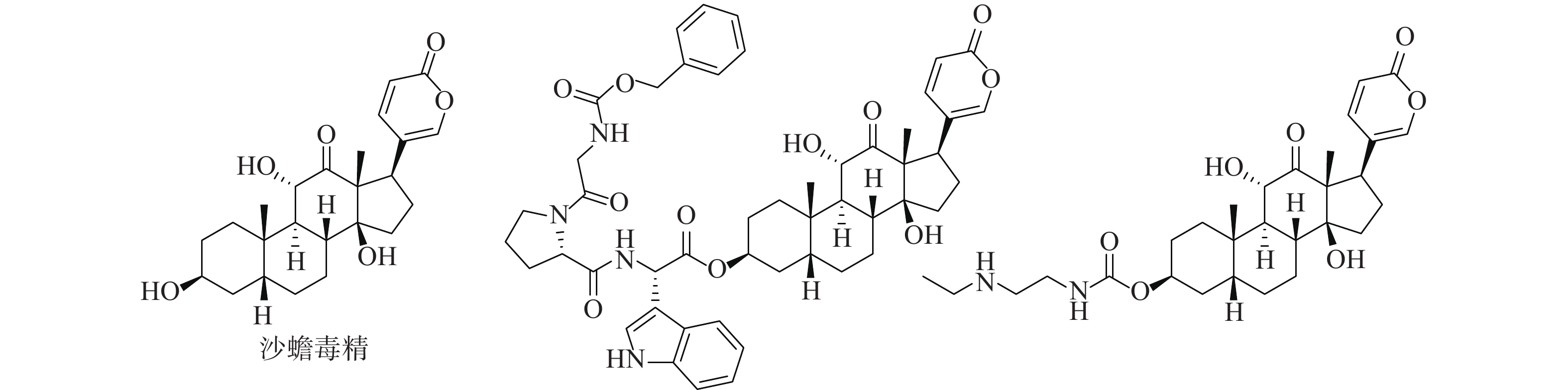
活性天然产物是药物研发重要的先导化合物来源之一,基于天然产物已成功开发出很多临床治疗药物。从我国传统中药中分离有效活性成分,已成为国内外发现先导化合物的一个有效途径。蟾蜍是我国特有中药材,具有解毒、消肿、止痛、强心利尿、抗癌等作用[1-3]。现代研究发现中药蟾蜍含有多种抗肿瘤活性成分,其中,主要化学成分之一的沙蟾毒精(图1)具有优秀的体内外抗肿瘤活性,但其心脏毒性使得沙蟾毒精治疗窗窄,无法作为抗肿瘤药物进行后续开发[4-7]。早期研究表明,沙蟾毒精的3位羟基对活性和毒性有较大影响[8]。Deng等对沙蟾毒精的3位进行了修饰,设计了一类成纤维细胞活化蛋白-α靶向沙蟾毒精前药衍生物,不仅在体内外显示出优异的抗肿瘤活性,而且降低了心脏毒性[9]。果德安等也对沙蟾毒精的3位羟基进行了结构修饰,设计得到一类3-氮取代氨基甲酸酯类衍生物,对A549、HCT116、Calu-6、PANC-1、SW620和NSCLC等6种细胞株均显示出优秀的活性,其IC50值在10 nmol/L以下[10]。因此,笔者设计3位酯基取代的沙蟾毒精衍生物,进一步探讨沙蟾毒精的构效关系,从中筛选获得高活性的候选化合物。
HTML
-
核磁共振仪为Bruker 300或600型,TMS为内标,CD3OD、DMSO-d6或者CDCl3为溶剂。化学位移(δ)和偶合常数(J)分别以×10−6(ppm)和Hz为单位。在API-3000LC-MS型质谱仪上完成ESI质谱。TLC分析所用硅胶板为GF254(青岛海洋化工有限公司)。硅胶柱层析采用60G硅胶(青岛海洋化工有限公司)。商品化溶剂皆为市售分析纯或化学纯。
-
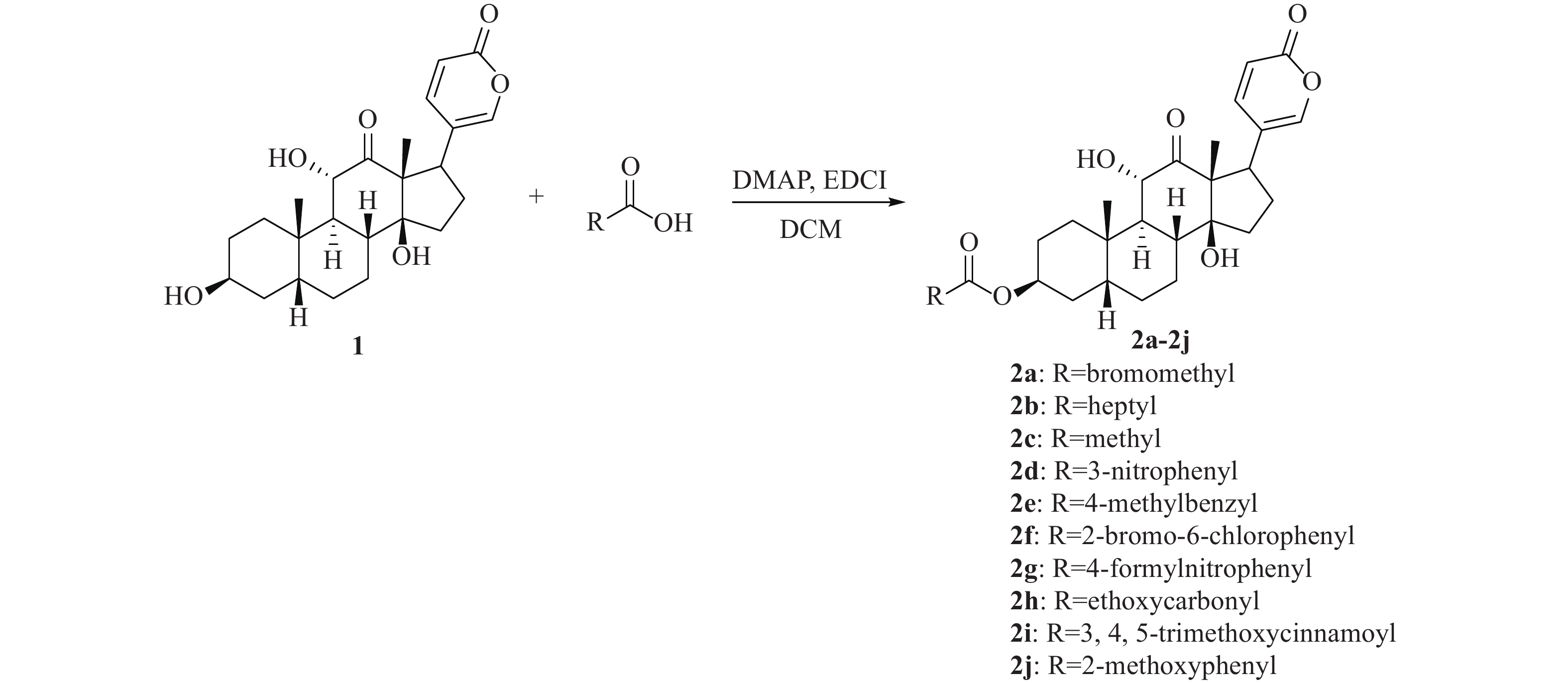
将沙蟾毒精(83 mg, 0.2 mmol)、酸(0.3 mmol)、DMAP(24.4 mg, 0.2 mmol)和5 ml干燥的二氯甲烷加入到25 ml烧瓶中,氩气保护下逐份加入EDCI(115 mg, 0.6 mmol)。室温反应过夜,反应完全后蒸去溶剂,残余物经柱色谱纯化后得目标产物2a−2j (洗脱剂为二氯甲烷∶甲醇=100∶1)。
2a: 白色固体,收率13.9%。1H NMR (500 MHz, CDCl3) δ: 7.68 (d, 1H), 7.36 (s, 1H), 6.28 (d,1H), 5.43 (s,1H), 5.18 (d,1H), 4.14 (d, 1H), 4.05 (d, 2H), 3.91 (q, 1H), 2.15 (d, 2H), 2.02~2.11 (m, 1H), 1.86 (m, 9H), 1.40 (s, 4H), 1.26 (d, 2H), 1.14 (s, 3H), 0.97 (s, 3H)。
2b: 白色固体,收率为55.5%。1H NMR: (CDCl3, 500 MHz) δ: 7.76 (d, 1H), 7.41 (s, 1H), 6.27 (d, 1H), 5.30 (s, 1H), 4.33 (d,1H), 4.11 (d,1H), 3.84 (s, 1H), 2.43 (d, 1H), 2.30 (t, 2H), 2.05~2.09 (m, 2H), 1.86~1.94 (m, 2H), 1.75~1.80 (m, 5H), 1.63~1.73 (m, 4H), 1.45 (d,1H), 1.33~1.44 (m,15H), 1.27 (s, 3H), 0.86~0.88 (m, 3H)。
2c: 白色固体,收率15.2%。1H NMR (500 MHz, CDCl3) δ: 7.74 (d, 1H), 7.40 (d, 1H), 6.30 (d, 1H), 5.08 (s, 1H), 4.35 (d, 1H), 4.11 (m, 1H), 3.84 (d, 1H), 2.45 (d, 1H), 2.08 (s, 3H), 1.65~1.92 (m, 11H), 1.46~1.53 (m, 1H), 1.26~1.42 (m, 5H), 1.21 (s, 3H), 0.92 (s, 3H)。
2d: 白色固体,收率46.7%。1H NMR: (CDCl3, 500MHz) δ: 8.85 (s, 1H), 8.40 (m, 2H), 7.62~7.77 (m, 2H), 7.41 (s, 1H), 6.29 (d, 1H), 5.38 (s, 1H), 4.36 (m, 1H), 4.13 (d, 1H), 3.86 (d, 1H), 2.56 (d, 1H), 1.72~2.24 (m, 11 H), 1.32~1.51 (m, 6H), 1.23(s, 3 H), 0.94 (s, 3H)。
2e: 白色固体,收率43.5%。1H NMR (500 MHz, CDCl3) δ: 7.66~7.74 (m, 1H), 7.37 (d, 1H), 7.08~7.20 (m, 3H), 6.27 (t,1H), 5.30 (s, 1H), 4.36~4.26 (m, 1H), 4.00~4.15 (m, 2H), 3.81 (m, 1H), 3.56 (d, 1H), 2.27~2.41 (m, 3H), 2.04 (m, 2H), 1.80~1.86 (m, 3H), 1.70~1.79 (m, 4H), 1.64 (s, 3H), 1.26~1.42 (m, 6H), 1.18 (s, 1H), 1.13 (s, 1H), 1.01 (d, 1H), 0.91 (d, 3H)。
2f: 白色固体,收率50.3%。1H NMR (500 MHz, CDCl3) δ: 7.94 (d, 1H), 7.72 (d, 1H), 7.53 (d, 1H), 7.40 (s, 1H), 7.32 (d, 1H), 6.29 (d, 1H), 5.36 (s, 1H), 5.30 (s, 1H), 4.35 (t, 1H), 4.06~4.16 (m, 1H), 3.85 (d, 1H), 2.51 (d, 1H), 1.97~2.13 (m, 3H), 1.74~1.89 (m, 8H), 1.45~1.54 (m, 1H), 1.31~1.45 (m, 3H), 1.25 (s, 1H), 1.21 (s, 3H), 0.93 (s, 3H)。
2g: 白色固体,收率42.6%。1H NMR (500 MHz, CDCl3) δ: 10.11 (s, 1H), 8.20 (d, 2H), 7.97 (d, 2H), 7.72 (d, 1H), 7.40 (s, 1H), 6.29 (d, 1H), 5.37 (s, 1H), 5.30 (s, 1H), 4.36 (m, 1H), 4.12 (t, 1H), 3.81~3.93 (m, 1H), 2.55 (d, 1H), 2.08 (t, 2H), 1.84~2.00 (m, 4H), 1.76~1.84 (m, 5H), 1.61 (s, 3H), 1.38 (d, 3H), 1.25 (s, 3H), 0.93 (s, 3H)。
2h: 白色固体,收率12.5%。1H NMR: (CDCl3, 500MHz) δ: 7.73 (m, 1H), 7.40 (s, 1H), 6.27 (d, 1H), 5.23 (s, 1H), 4.35(q, 2H), 4.36~4.40 (m, 1H), 4.04~4.14 (m, 1H), 2.46 (d, 1H), 1.69~2.26(m, 10H), 1.57 (d, 1H), 1.22~1.50 (m, 8H), 1.20 (s, 3H), 0.91 (s, 3H)。
2i: 白色固体,收率52.3%。1H NMR (500 MHz, CDCl3) δ: 7.74 (m, 1H), 7.61 (d, 1H), 7.42 (s, 1H), 6.79 (s, 2H), 6.38 (d, 1H), 6.32 (d, 1H), 5.32 (s, 1H), 5.23 (s, 1H), 4.37 (m, 1H), 4.08~4.19 (m, 1H), (3.92 (d, 9H), δ3.87 (d, 1H), 2.51 (d, 1H), 1.84 (m,, 10H), 1.40 (t, 3H), 1.28 (s, 3H), 1.25 (s, 3H), 0.95 (s, 3H)。
2j: 白色固体,收率39.2%。1H NMR (500 MHz, CDCl3) δ: 7.84 (d, 1H), 7.74 (m, 1H), 7.50 (t, 1H), 7.42 (s, 1H), 7.02 (t, 2H), 6.32 (d, 1H), 5.35 (s, 1H), 4.37 (m, 1H), 4.18~4.09 (m, 1H), 3.92 (d, 3H), 3.87 (d, 1H), 2.52 (d,, 1H), 2.09 (d, 1H), 1.78~2.00 (m, 7H), 1.59 (s, 3H), 1.28~1.46 (m, 6H), 1.25 (s, 3H), 0.96 (s, 3H)。
-
选用人乳腺癌细胞MCF-7、人肝癌细胞Bel7404和人肠癌细胞HCT116,均由海军军医大学药学院冻存和传代。在37 ℃、5 % CO2条件下,用含有10 %的胎牛血清、1 %的青霉素和链霉素的DMEM培养基中传代培养细胞。弃去培养皿中的上层培养基,用PBS洗细胞2次,再加入胰酶,放入培养基中消化1~2 min,待细胞脱壁后,再加入新的培养基,轻轻吹打,使细胞完全脱落,待细胞入5 ml新的培养基,轻轻吹打,用细胞计数法计算细胞浓度,然后接种于96孔板中。将接种完的96孔板放置于37 ℃、5 %的CO2培养箱中孵育过夜,次日细胞即可贴壁。按照不同的实验设计加入不同浓度的药物,每组设3~4个复孔,每孔加入10 µl相应浓度的药物,再将96孔板放入培养箱继续培养。活性测试采用10 % CellTiterTM Blue(Promega, Cat.# G8081/2, US)试剂盒,并在37 ℃、5 % CO2环境下孵育1 h。用酶标仪(Bio Tek, US)测量530/40 nm和590/35 nm处荧光值,计算公式:
细胞存活率 (%) = [(As−Ab)/(Ac−Ab)]×100%。
其中,As: 实验组(含有细胞、荧光试剂和化合物的培养基);Ac: 对照组(含有细胞和荧光试剂,不含化合物的培养基);Ab: 空白组(不含细胞、化合物的培养基)。
2.1. 化合物2a−2j的合成方法
2.2. 体外抗肿瘤活性测试
-
目标化合物通过各类酸和沙蟾毒精发生酯化反应得到,收率为12.5%~55.5%,合成路线见图2。从反应收率上看,芳香族酯类衍生物收率高于脂肪族类酯类衍生物。例如,化合物2c为乙酸酯衍生物,收率仅为15.2%,而化合物2e为对甲基苄酯衍生物,收率为43.5%。在反应中还发现,水分对反应有较大影响。因此,反应溶剂需要干燥处理,并且反应体系还需保持在无水条件下,才能保证反应顺利进行。
-
课题组选择3种肿瘤细胞株(MCF-7、Bel7404和HCT116),以沙蟾毒精为阳性对照,采用CellTiter法开展了体外抗肿瘤活性研究,结果见表1。可以看出,3-沙蟾毒精酯类衍生物均具有优异的体外抗肿瘤活性。对MCF-7细胞株,化合物2a、2c和2j显示出高于阳性对照药沙蟾毒精的活性。对Bel7404细胞株,所有化合物均显示出优异的活性,其中,化合物2a、2b、2d和2e活性最好,其IC50值均在数个纳摩尔,相比沙蟾毒精提高近1000倍。对HCT116细胞株,化合物2e活性最高,其IC50值为4.5 nmol/L,相比沙蟾毒精提高了3.7倍,化合物2b和2c与阳性药相当。
化合物 MCF-7 Bel 7404 HCT 116 2a 0.0917 0.0040 0.0489 2b 0.2403 0.0024 0.0163 2c 0.0471 0.0479 0.0173 2d 0.1817 0.0048 0.0279 2e 0.1910 0.0040 0.0045 2f 0.1982 0.0203 0.2043 2g 0.1887 0.0212 0.0605 2h 0.1616 0.5365 0.0302 2i 0.1418 0.0252 0.0488 2j 0.0968 0.0168 0.0258 沙蟾毒精 0.1101 4.5440 0.0167 整体而言,脂肪族酯类化合物活性高于芳香族酯类化合物,例如对MCF-7细胞株,溴乙酸酯衍生物2a的IC50值为91.7 nmol/L,而芳香族酯类化合物除2j外,活性均在100 nmol/L以上。
3.1. 化学合成
3.2. 体外抗肿瘤活性研究
-
基于活性天然产物沙蟾毒精骨架,在其3位引入酯基基团,设计合成出10个3-沙蟾毒精酯类衍生物。体外抗肿瘤活性研究发现,所有的化合物对3种肿瘤细胞株MCF-7、Bel7404和HCT116均显示出优异的抗肿瘤活性。其中,化合物2a活性最好,对3种肿瘤细胞株的IC50值均在100 nmol/L以下,可作为抗肿瘤候选化合物进行进一步研究。








 DownLoad:
DownLoad: