-
外科手术部位感染(SSI)是医疗保健相关感染的常见原因[1],大多数SSI发生的平均时间为术后12 d[2]。耐甲氧西林金黄色葡萄球菌(MRSA)是指对已经批准的所有β内酰胺类抗菌药物有交叉耐药的金黄色葡萄球菌[3]。感染MRSA外科伤口较严重的患者,建议静脉输注糖肽类、利奈唑胺或达托霉素治疗[3]。因临床应用万古霉素经验丰富,为胃肠外首选[4]。但其治疗窗窄、不良反应多、血药浓度影响因素较多等特点,根据患者临床情况实施治疗药物监测(TDM)和个体化用药显得尤为重要。笔者介绍1例临床药师对足损伤术后感染MRSA患者进行药学监护的体会。
-
患者,男,17岁,体重60 kg,2020-05-04因“车祸致左足开放性外伤,出血30 min”入院,主要诊断为足部开放性损伤伴骨折、足部损伤、跟腱断裂。05-05行Ⅰ期清创+骨折切开复位内固定+跟腱修复+甲床修复术,术后头孢呋辛预防48 h。05-07手术切口感染,给予头孢呋辛治疗,05-08行Ⅱ期清创+创面封闭式负压引流术。05-11出现发热,体温最高38.2 ℃,炎性指标异常升高,换用万古霉素1.0 g ivgtt q12h。05-12切口分泌物培养+药敏示:MRSA、多重耐药,万古霉素敏感。05-19行Ⅲ期左足清创+带蒂皮瓣转移修复+跟腱修复术,头孢呋辛1.5 g ivgtt q8h预防感染,术后次日切口感染,体温最高38.7 ℃,换用万古霉素1.0 g ivgtt q12h抗感染治疗。其他治疗:Ⅰ~Ⅲ期术后常规氟比洛芬酯注射液镇痛3 d,Ⅲ期术后地塞米松抗炎、甘油果糖消肿、氟比洛芬酯注射液镇痛3 d,改为双氯芬酸钠胶囊。05-22万古霉素血药浓度检测为4.08 μg/ml,05-23调整万古霉素剂量为1.0 g ivgtt q8h,停用甘油果糖、地塞米松,05-26停用双氯芬酸钠胶囊。05-25、05-29复测万古霉素血药浓度为8.41和10.23μg/ml。在增加万古霉素剂量的次日上午,患者出现面部潮红症状,调慢滴速,症状缓解至消失,未再出现类似ADR。05-29出现急性肝损伤,停用万古霉素,给予甘草酸二铵保肝治疗1周,06-04切口恢复良好,准予出院。两周后随访,肝功能恢复正常。
-
术后第3天,患者发生足部切口感染,且为入院48 h后发生,予头孢呋辛治疗,体温、炎性指标未得到明显改善,临床药师高度怀疑为医院获得性MRSA。根据《哈里森感染病学》皮肤软组织感染治疗,对于社区或医院获得性金黄色葡萄球菌感染,对β-内酰胺类抗生素无应答,需换用抗MRSA药物治疗。临床药师建议换用万古霉素1.0 g ivgtt q12h治疗,医师采纳意见,当晚患者体温恢复正常。Ⅲ期手术预防用药,临床药师针对患者本次住院已检出MRSA,且近半年骨科MRSA检出率为62.5%,建议调整为万古霉素[5],但医师未采纳药师建议。
-
术后第3天,出现切口红肿热痛、大量渗出、发热,诊断外科切口感染明确,选择万古霉素。临床药师根据未接受血液透析的成人胃肠外万古霉素剂量推荐表[6],结合患者体重、非重症感染、肌酐清除率指标,推荐初始剂量为1.0 g ivgtt q12 h。
-
患者05-29因出现急性肝损伤,根据《万古霉素临床应用专家共识》[4]推荐疗程为7~14 d,患者用药9 d,临床药师根据患者临床症状无明显红肿热痛,炎性指标趋于正常,可停用抗菌药物治疗。
-
05-19行Ⅲ期术后次日,切口出现感染征象,给予万古霉素抗感染治疗。05-20至05-23患者切口感染部位症状缓解不明显,一直低热状态。临床药师建议行万古霉素血药浓度监测,同时建议停用可能影响万古霉素血药浓度的甘油果糖,并参考《万古霉素个体化给药临床药师指引》[7]调整其剂量为1.0 g ivgtt q8 h。万古霉素剂量调整前血药浓度为4.08 μg/ml,调整后复测2次血药浓度分别为8.41、10.23 μg/ml。通过停用甘油果糖、TDM、调整万古霉素剂量,患者血药浓度显著升高,并达到有效治疗浓度[4]。
影响成年患者体内万古霉素的浓度因素包括年龄、中重度外周水肿、肾功能异常、肥胖及肾功能亢进等因素[8-9]。患者术后切口部位严重水肿,炎性反应明显,在一定程度上会对血液内稳态产生一定影响,导致机体代谢药物的能力也受到一定影响。万古霉素联合应用甘油果糖可能会严重影响其血药浓度,有研究显示[10],渗透性药物能促进万古霉素在体内的清除和排泄,令血药浓度降低。本例患者本身存在严重组织水肿,同时使用渗透性药物甘油果糖,可能是影响万古霉素血药浓度过低的原因。肾功能亢进的原因包括烧伤、粒细胞缺乏伴发热、脓毒血症、创伤、蛛网膜下隙出血等[11]。该患者严重创伤入院,肌酐清除率为145.94 ml/min,存在肾功能亢进,可能在一定程度上导致患者万古霉素血药浓度过低。
-
患者增加万古霉素使用剂量至1.0 g ivgtt q8 h,次日上午出现面部、颈部微潮红。临床药师结合患者用药史,采用诺氏评估量表评价ADR关联性,评分为7分,考虑很可能为万古霉素相关性引起的红人综合征(RMS)。RMS是在万古霉素输注期间或输注结束后立即出现的潮红,由组胺介导。组胺释放量一般与输注万古霉素的剂量和输注速度有关[12]。该患者在未调整万古霉素剂量之前,输注时间控制在60 min左右,一直未发生相关不良反应。临床药师建议先以给药速率≤10 mg/min输注(或1.0 g,输注时间≥100 min)[12],并密切监护。结果减慢滴速后,患者潮红症状消退,并未再出现RMS症状。
-
对于肾功能正常,合并肾损伤药物且疗程较长的患者,应密切监测肾功能[4]。本例患者在Ⅰ~Ⅲ期术后,常规使用非甾体类药物镇痛,为避免增加肾损伤可能性,结合患者疼痛评分3分,临床药师建议尽早停用非甾体类药物,或换用肾功能损伤最小的阿片类镇痛药[13],医师采纳该意见。
-
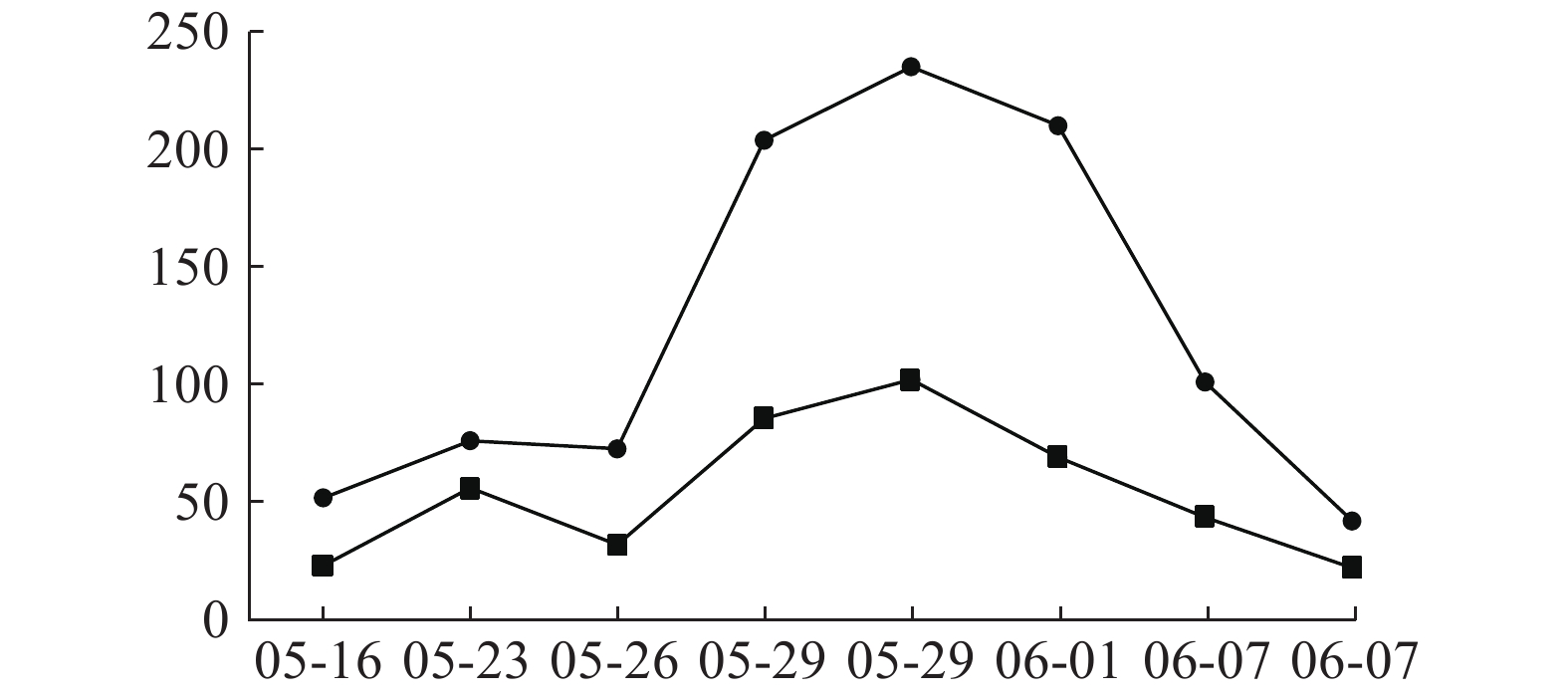
患者入院时肝功能正常,05-09开始用万古霉素1.0 g ivgtt q12 h治疗,05-16查肝功示ALT 51.7 U/L,05-18停用;05-20因病情需要再次使用万古霉素1.0 g ivgtt q12 h治疗,05-23调整剂量为1.0 g ivgtt q8 h。05-26复查肝功示ALT 72.6.00 U/L,AST 31.8 U/L,出现转氨酶进行性小幅升高,继续万古霉素抗MRSA治疗,符合《EASL:药物性肝损伤的临床实践指南》[14]。05-29两次复查肝功示:ALT 203.8 U/L,AST 85.5 U/L;ALT 235.00 U/L,AST 102.00 U/L,提示ALT≥3倍正常值上限,出现急性肝损伤,停用万古霉素,给予甘草酸二铵保肝治疗,06-04出院并停用甘草酸二铵。06-25随访肝功能完全恢复正常。万古霉素治疗前后ALT、AST指标变化见图1。
临床药师以诺氏评估量表作ADR关联性评价,评分为9分,患者肝损害与万古霉素不良反应发生有时间上的关联,高度可能为万古霉素引起肝功能损伤。万古霉素导致肝损伤报道例数较少[15-18],以血清转氨酶升高为主[15-18],临床表现可为无症状性肝损伤或急性肝损伤。本例患者系无症状性急性肝损伤,提示关注万古霉素长疗程治疗中肝损伤ADR。
-
临床药师作为医疗团队中的一员,在药物使用过程中,尤其在药物浓度监测和药学监护方面发挥了重要作用。在该患者治疗过程中,临床药师查阅指南、文献及药品说明书,协助医师做好抗感染治疗方案的制定,并结合临床疗效,加强抗感染治疗的药学监护,分析治疗效果不佳原因,并有针对性地提出建议与改进措施,包括调整合并用药及药物剂量,避免和减少了万古霉素相关的RMS、肝肾功能损伤不良反应对患者进一步伤害,使患者临床获益更多。
The pharmaceutical care by clinical pharmacists for a foot injury patient with postoperative MRSA infection
doi: 10.12206/j.issn.1006-0111.202102004
- Received Date: 2021-02-04
- Rev Recd Date: 2021-06-10
- Available Online: 2023-11-06
- Publish Date: 2022-05-25
-
Key words:
- clinical pharmacist /
- vancomycin /
- MRSA /
- pharmaceutical care
Abstract:
| Citation: | LUO Jingjing, WU Xinan, XUE Gang, XIE Zhengdong, YE Jianbo, RONG Chengting. The pharmaceutical care by clinical pharmacists for a foot injury patient with postoperative MRSA infection[J]. Journal of Pharmaceutical Practice and Service, 2022, 40(3): 286-288. doi: 10.12206/j.issn.1006-0111.202102004 |








 DownLoad:
DownLoad: